Supramolecular assemblies in mononuclear Zn(II) and dinuclear Co(II) coordination compounds involving pyridines and methyl benzoates: Antiproliferative evaluation and theoretical studies
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Two new metal-organic coordination compounds of Zn(II) and Co(II) viz. [Zn(3-CNpy)2(H2O)4](4-Mebz)2 (1)and [Co2(μ-H2O)(py)4(μ-2-Mebz)2(2-Mebz)2] (2) (3-CNpy = 3-cyanopyridine, 4-Mebz = 4-methylbenzoate, 2-Mebz = 2-methylbenzoate, py = pyridine) have been synthesized and characterized using single crystal X-ray study, elemental analysis, FT-IR, electronic spectroscopy and thermogravimetric analysis (TGA). Compound 1 crystallizes as a mononuclear Zn(II) complex, whereas compound 2 is a water and 2-Mebz bridged dinuclear coordination compound of Co(II). Crystal structure analysis of compound 1 highlights the presence of aromatic π stacking, π–π(CN) and O⋯O dichalcogen bonding interactions along with interesting charge-assisted hydrogen bonding interactions which together stabilize the layered assembly of the compound. Again, antiparallel π-stacking combined with C![]() H⋯π and C
H⋯π and C![]() H⋯O hydrogen bonding interactions stabilize the crystal packing of compound 2. To characterize and rationalize these interactions, we utilized various computational tools, including DFT, MEP surface calculations, QTAIM analysis and NCIplot. Theoretical studies confirm the existence of structure-directing π–π(CN), π-stacking and H-bonding interactions involving strong O
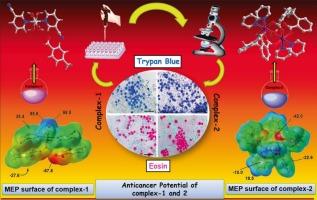
H⋯O hydrogen bonding interactions stabilize the crystal packing of compound 2. To characterize and rationalize these interactions, we utilized various computational tools, including DFT, MEP surface calculations, QTAIM analysis and NCIplot. Theoretical studies confirm the existence of structure-directing π–π(CN), π-stacking and H-bonding interactions involving strong O![]() H⋯O contacts (−42.4 kcal/mol) in 1. Significantly negative dimerization energies (−29.0 kcal/mol) were obtained for 2. The in vitro antiproliferative effects of compounds 1 and 2 were assessed on Dalton's lymphoma (DL) malignant cancer cell lines and normal PBMC cells using MTT, Trypan blue and eosin staining assays, demonstrating higher cytoxicity of compound 2 than compound 1. The IC50 values (DL) obtained for compounds 1 and 2 were 62.7μΜ and 22.7μΜ respectively. Molecular Docking studies of the compounds with antiapoptotic proteins showed that compound 2 interacted more effectively [binding score: −93.18 kcal/mol−1 (MCL-1)] than compound 1 [binding score: 67.61 kal/mol−1 (MCL-1)].
H⋯O contacts (−42.4 kcal/mol) in 1. Significantly negative dimerization energies (−29.0 kcal/mol) were obtained for 2. The in vitro antiproliferative effects of compounds 1 and 2 were assessed on Dalton's lymphoma (DL) malignant cancer cell lines and normal PBMC cells using MTT, Trypan blue and eosin staining assays, demonstrating higher cytoxicity of compound 2 than compound 1. The IC50 values (DL) obtained for compounds 1 and 2 were 62.7μΜ and 22.7μΜ respectively. Molecular Docking studies of the compounds with antiapoptotic proteins showed that compound 2 interacted more effectively [binding score: −93.18 kcal/mol−1 (MCL-1)] than compound 1 [binding score: 67.61 kal/mol−1 (MCL-1)].
涉及吡啶和苯甲酸甲酯的单核Zn(II)和双核Co(II)配位化合物中的超分子组装:抗增殖评价和理论研究
合成了Zn(II)和Co(II)两种新的金属有机配位化合物[Zn(3-CNpy)2(H2O)4](4- mebz)2(1)和[Co2(μ-H2O)(py)4(μ-2-Mebz)2(2- mebz)2] (2) (3-CNpy = 3-氰吡啶,4- mebz = 4-甲基苯甲酸酯,2- mebz = 2-甲基苯甲酸酯,py =吡啶),并采用单晶x射线研究、元素分析、FT-IR、电子能谱和热重分析(TGA)对其进行了表征。化合物1结晶为单核Zn(II)配合物,而化合物2是Co(II)的水和2- mebz桥接双核配位化合物。化合物1的晶体结构分析强调了芳香π堆积,π -π (CN)和O⋯O二碳键相互作用以及有趣的电荷辅助氢键相互作用的存在,这些相互作用共同稳定了化合物的层状组装。再一次,反平行π堆积结合CH⋯π和CH⋯O氢键相互作用稳定了化合物2的晶体堆积。为了描述和合理化这些相互作用,我们使用了各种计算工具,包括DFT, MEP表面计算,QTAIM分析和NCIplot。理论研究证实,在1中存在指向结构的π -π (CN)、π-堆叠和涉及强OH⋯O接触(−42.4 kcal/mol)的氢键相互作用。2的二聚化能显著为负(- 29.0 kcal/mol)。采用MTT、台盼蓝和伊红染色法对化合物1和2对道尔顿淋巴瘤(DL)恶性癌细胞系和正常PBMC细胞的体外抗增殖作用进行了评价,结果表明化合物2的细胞毒性高于化合物1。化合物1和2的IC50值(DL)分别为62.7μΜ和22.7μΜ。化合物与抗凋亡蛋白的分子对接研究表明,化合物2比化合物1更有效地相互作用[结合分数:−93.18 kcal/mol−1 (MCL-1)][结合分数:67.61 kal/mol−1 (MCL-1)]。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


