Theoretical study of excited-state dynamics and pH detection of 2,2’-binaphthol chiral probes
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
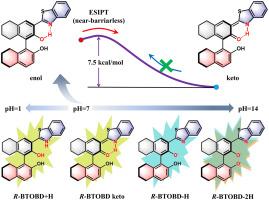
Chiral probes with excited state intramolecular proton transfer (ESIPT) show significant potential in bioimaging, environmental detection, etc. The chiral 2,2′-binaphthol derivative (R-BTOBD) probe demonstrates pH-dependent luminescence characteristics. In this paper, hydrogen bond dynamics, ESIPT process, and spectral attribution of R-BTOBD are investigated by the time-dependent density functional theory. Results show that the thermodynamically favored enol configuration undergoes charge transfer excitation, with photoinduced strengthening of the O![]() H⋯N hydrogen bond facilitating a near-barrierless ESIPT process. The protonation/deprotonation configurations of R-BTOBD and its spectra studies reveal its remarkable pH-response mechanism. This study provides theoretical guidance for the design of chiral probes.
H⋯N hydrogen bond facilitating a near-barrierless ESIPT process. The protonation/deprotonation configurations of R-BTOBD and its spectra studies reveal its remarkable pH-response mechanism. This study provides theoretical guidance for the design of chiral probes.
2,2′-联萘酚手性探针激发态动力学和pH检测的理论研究
具有激发态分子内质子转移(ESIPT)的手性探针在生物成像、环境检测等领域具有重要的应用前景。手性2,2 ' -联萘酚衍生物(R-BTOBD)探针具有ph依赖的发光特性。本文利用时变密度泛函理论研究了R-BTOBD的氢键动力学、ESIPT过程和谱属性。结果表明,热力学上有利的烯醇构型经历了电荷转移激发,光诱导OH⋯N氢键的增强促进了近乎无障碍的ESIPT过程。R-BTOBD的质子化/去质子化构型及其光谱研究揭示了其显著的ph响应机制。该研究为手性探针的设计提供了理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


