Single-cell RNA sequencing reveals early cell dynamics of MSC-based therapy in long bone critical-size defects in mice
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
Bone defects resulting from various causes present significant challenges in obtaining robust bone healing. This clinical scenario is particularly difficult in cases involving large bone defects, often leading to delayed union or non-union. Autogenous bone graft is the gold standard, but it is limited by the quantity and quality of available bone. Mesenchymal stem cells (MSCs) have shown promise in enhancing bone defect healing; however, the mechanisms by which MSCs modify the local bone microenvironment and interact with other cells early in the healing process are not fully understood. Elucidating and modulating the early biological events relevant to the healing of bone defects could lead to novel therapies to obtain a more expeditious and complete outcome.
Methods
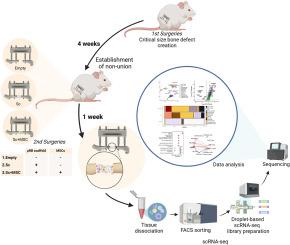
Critical-size femoral defects were created in 10 to 12-week-old BALB/c male mice and fixed with an external fixation device. Four weeks after the generation of the defect, secondary surgeries were performed. Mice were randomized into three groups based on the secondary surgery: Empty group - surgery was performed without implanting scaffolds or cells. Sc group - a 2 mm diameter cylindrical microribbon (μRB) scaffold was implanted into the defect site. Sc + MSC group - a scaffold embedded with MSCs was implanted into the bone defect site. One week after the secondary surgeries, the entire tissue within the bone defect site was harvested for single-cell RNA sequencing (scRNA-seq).
Results
Uniform manifold approximation and projection (UMAP) plots with quality filtered cells from three groups were used to identify the cell distributions in the defects. We identified thirteen populations and annotated each cluster using UMAP with Louvain clustering on combined single cells of three groups based on marker gene expression. Different cell compositions were revealed, especially the proportion of various types of immune cells in the Sc vs Sc + MSC groups. MSCs and osteoblastic lineage cells (MSC/Osteo), and osteoclasts were almost exclusively found in the Sc + MSC group. Differential gene expression and pathway analysis in major cell populations identified immune cell changes and inflammatory changes in the presence of implanted MSCs. Cell–cell communications revealed a greater number of interactions between different cell types in the Sc and Sc + MSC groups. More interactions among MSCs, macrophages, and T cells were observed in Sc + MSC groups. MSC demonstrated the highest outgoing interaction strength in all groups.
Conclusions
In the critical-size bone defect model, a combination of MSCs with μRB scaffolds showed an increased presence of mesenchymal lineage cells and promoted the further recruitment of macrophages and osteoclasts at 1 week. This alteration in the local immune landscape and microenvironment could enhance the cellular dynamics of critical cell populations that are important to osteogenesis. Optimizing this cellular crosstalk early in the healing process could potentially augment MSC-based therapies for subsequent bone regeneration of critical-size bone defects.
The translational potential of this article
The results of our study provide a detailed transcriptional roadmap for local immune modulation by MSCs in scaffolds, supporting the optimization of robust strategies for MSC-based treatments of long bone critical-size defects in future clinical applications.
单细胞RNA测序揭示了基于msc的治疗小鼠长骨临界尺寸缺陷的早期细胞动力学
背景:各种原因导致的骨缺损对获得强健的骨愈合提出了重大挑战。这种临床情况在涉及大骨缺损的病例中尤其困难,通常导致延迟愈合或不愈合。自体骨移植是金标准,但受可用骨的数量和质量的限制。间充质干细胞(MSCs)已显示出促进骨缺损愈合的前景;然而,MSCs在愈合过程早期改变局部骨微环境并与其他细胞相互作用的机制尚不完全清楚。阐明和调节与骨缺损愈合相关的早期生物学事件可能会导致新的治疗方法,以获得更快速和完整的结果。方法采用10 ~ 12周龄BALB/c雄性小鼠建立临界大小的股骨缺损,用外固定装置固定。缺损发生4周后,进行二次手术。小鼠根据二次手术随机分为三组:空组-手术不植入支架或细胞。Sc组:在缺损部位植入直径为2mm的圆柱形微带(μRB)支架。Sc + MSC组-将MSCs包埋支架植入骨缺损部位。二次手术后一周,采集骨缺损部位内的整个组织进行单细胞RNA测序(scRNA-seq)。结果采用均匀流形逼近和投影(UMAP)图,对三组细胞进行质量过滤,识别缺陷中的细胞分布。我们鉴定了13个群体,并基于标记基因表达对三组组合单细胞使用UMAP和Louvain聚类对每个聚类进行注释。在Sc组和Sc + MSC组中发现了不同的细胞组成,特别是各种类型的免疫细胞的比例。MSC和成骨细胞谱系细胞(MSC/Osteo)以及破骨细胞几乎只存在于Sc + MSC组。主要细胞群的差异基因表达和通路分析鉴定了移植MSCs存在下的免疫细胞变化和炎症变化。细胞间通讯显示Sc和Sc + MSC组中不同细胞类型之间有更多的相互作用。在Sc + MSC组中,MSCs、巨噬细胞和T细胞之间的相互作用更多。MSC在所有组中表现出最高的外向互动强度。结论在临界尺寸骨缺损模型中,MSCs与μRB支架联合使用可增加间充质谱系细胞的存在,并促进巨噬细胞和破骨细胞的进一步募集。这种局部免疫景观和微环境的改变可以增强对成骨很重要的关键细胞群的细胞动力学。在愈合过程的早期优化这种细胞串扰可能潜在地增强基于msc的治疗方法,用于随后的临界大小骨缺损的骨再生。我们的研究结果为MSCs在支架中的局部免疫调节提供了详细的转录路线图,支持在未来临床应用中优化基于MSCs的长骨临界尺寸缺陷治疗的稳健策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


