HIC2 suppresses glioblastoma progression via transcriptional repression of SEMA3A and inhibition of TGF-β signaling
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Glioblastoma (GBM), the most aggressive primary brain tumor, is associated with dismal clinical outcomes and a critical lack of actionable therapeutic targets. Herein, we report that Hypermethylated in Cancer 2 (HIC2) is significantly downregulated in GBM tissues. In vitro, ectopic overexpression of HIC2 markedly suppresses GBM cell proliferation, invasion, and migration, while in vivo, it substantially inhibits tumor growth and prolongs survival in an orthotopic xenograft model (p < 0.01). Furthermore, HIC2 induces G0/G1 cell cycle arrest and robustly promotes apoptosis. Mechanistically, HIC2 directly binds to the promoter region of Semaphorin 3A (SEMA3A), repressing its transcriptional activity. This transcriptional repression subsequently attenuates TGF-β signaling by diminishing Smad2/3 phosphorylation, a critical regulatory node of this pathway. Collectively, our findings elucidate a previously unrecognized tumor-suppressive mechanism wherein HIC2 inhibits GBM progression through modulation of the SEMA3A-TGF-β signaling axis.
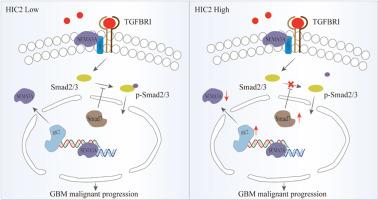
HIC2通过转录抑制SEMA3A和抑制TGF-β信号传导抑制胶质母细胞瘤的进展
胶质母细胞瘤(GBM)是最具侵袭性的原发性脑肿瘤,其临床结果令人沮丧,并且严重缺乏可行的治疗靶点。在此,我们报告了癌2中的高甲基化(HIC2)在GBM组织中显著下调。在体外,异位过表达HIC2可显著抑制GBM细胞的增殖、侵袭和迁移,而在体内,在原位异种移植模型中,HIC2可显著抑制肿瘤生长并延长存活时间(p < 0.01)。此外,HIC2还能诱导G0/G1细胞周期阻滞,促进细胞凋亡。在机制上,HIC2直接结合信号蛋白3A (SEMA3A)的启动子区域,抑制其转录活性。这种转录抑制随后通过减少Smad2/3磷酸化(该途径的关键调控节点)来减弱TGF-β信号。总的来说,我们的研究结果阐明了一种以前未被认识到的肿瘤抑制机制,其中HIC2通过调节SEMA3A-TGF-β信号轴抑制GBM进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


