Cytoskeletal disruption-induced calcium dysregulation drives cell death in anti-IgLON5 disease
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Anti-IgLON5 disease is an autoimmune encephalitis with more chronic presentation including memory decline, sleep disorder, bulbar symptoms and movement disorder. Post-mortem brains of patients with anti-IgLON5 disease show neurodegeneration with tau deposition sparking interest in this ‘acquired tauopathy’ as a disease model for neurodegeneration, yet mechanisms of neurodegeneration remain unknown. Using a reductionist human iPSC-derived neuron-antibody model, we applied proteomics approach, electrophysiology and live cell imaging. iNeurons treated with anti-IgLON5 IgG presented with cytoskeletal disruption along with tau depositions, which correlated with endophenotypes. Accompanying calcium dysregulation was driven by impaired ER refill and mitochondrial dysfunction leading to cell death. Analogous cytoskeletal disruption is also reflected in the serum of treatment naïve patients using OLink proteomics. These findings provide insight into anti-IgLON5 disease pathology and pinpoint downstream signalling events of direct antibody-neuron interactions, which involve novel targets such as cytoskeletal disruption along with calcium dysregulation.
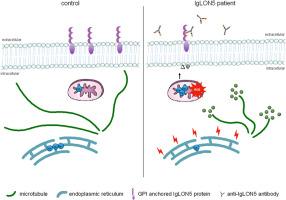
抗iglon5疾病中细胞骨架破坏诱导的钙失调驱动细胞死亡
抗iglon5疾病是一种慢性表现的自身免疫性脑炎,包括记忆力下降、睡眠障碍、球症状和运动障碍。抗iglon5疾病患者的死后大脑显示出神经变性和tau沉积,这引发了人们对这种“获得性tau病”作为神经变性疾病模型的兴趣,但神经变性的机制仍然未知。使用还原人ipsc衍生的神经元抗体模型,我们应用蛋白质组学方法,电生理学和活细胞成像。用抗iglon5 IgG处理的神经元出现细胞骨架破坏和tau沉积,这与内表型相关。伴随的钙失调是由内质网再填充受损和线粒体功能障碍导致细胞死亡所驱动的。类似的细胞骨架破坏也反映在使用OLink蛋白质组学治疗naïve患者的血清中。这些发现提供了对抗iglon5疾病病理的深入了解,并确定了直接抗体-神经元相互作用的下游信号事件,其中涉及新的靶点,如细胞骨架破坏和钙调节失调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


