Cellular pan-chain acyl-CoA profiling reveals SLC25A42/SLC25A16 in mitochondrial CoA import and metabolism
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
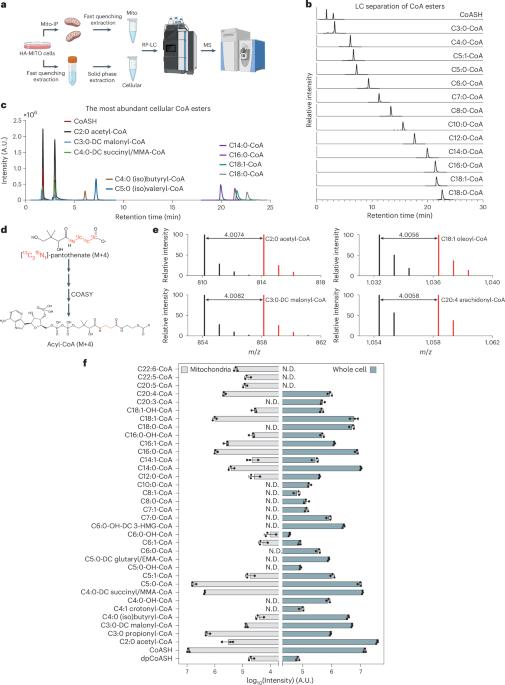
The essential cofactor coenzyme A (CoASH) and its thioester derivatives (acyl-CoAs) have pivotal roles in cellular metabolism. However, the mechanism by which different acyl-CoAs are accurately partitioned into different subcellular compartments to support site-specific reactions, and the physiological impact of such compartmentalization, remain poorly understood. Here, we report an optimized liquid chromatography–mass spectrometry-based pan-chain acyl-CoA extraction and profiling method that enables a robust detection of 33 cellular and 23 mitochondrial acyl-CoAs from cultured human cells. We reveal that SLC25A16 and SLC25A42 are critical for mitochondrial import of free CoASH. This CoASH import process supports an enriched mitochondrial CoA pool and CoA-dependent pathways in the matrix, including the high-flux TCA cycle and fatty acid oxidation. Despite a small fraction of the mitochondria-localized CoA synthase COASY, de novo CoA biosynthesis is primarily cytosolic and supports cytosolic lipid anabolism. This mitochondrial acyl-CoA compartmentalization enables a spatial regulation of anabolic and energy-related catabolic processes, which promises to shed light on pathophysiology in the inborn errors of CoA metabolism. By developing a method that allows detection of transient, low-abundance acyl-CoA species, Liu et al. provide a thorough characterization of coenzyme A dynamics and subcellular partitioning.
细胞泛链酰基辅酶a分析显示SLC25A42/SLC25A16参与线粒体辅酶a的输入和代谢
必需辅酶A (CoASH)及其硫酯衍生物(酰基辅酶A)在细胞代谢中起着关键作用。然而,不同的酰基辅酶a被精确地划分到不同的亚细胞区室以支持位点特异性反应的机制,以及这种区室化的生理影响,仍然知之甚少。在这里,我们报告了一种优化的基于液相色谱-质谱的泛链酰基辅酶a提取和分析方法,可以从培养的人类细胞中检测出33种细胞和23种线粒体酰基辅酶a。我们发现SLC25A16和SLC25A42对于线粒体导入游离CoASH至关重要。这种CoASH输入过程支持丰富的线粒体CoA库和基质中CoA依赖途径,包括高通量TCA循环和脂肪酸氧化。尽管有一小部分线粒体定位的CoA合成酶COASY,但从头合成CoA主要是细胞质内的,并支持细胞质内的脂质合成代谢。这种线粒体酰基辅酶a区区化使合成代谢和能量相关分解代谢过程的空间调节成为可能,这有望阐明辅酶a代谢先天性错误的病理生理学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


