Common genetic variants modify disease risk and clinical presentation in monogenic diabetes
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
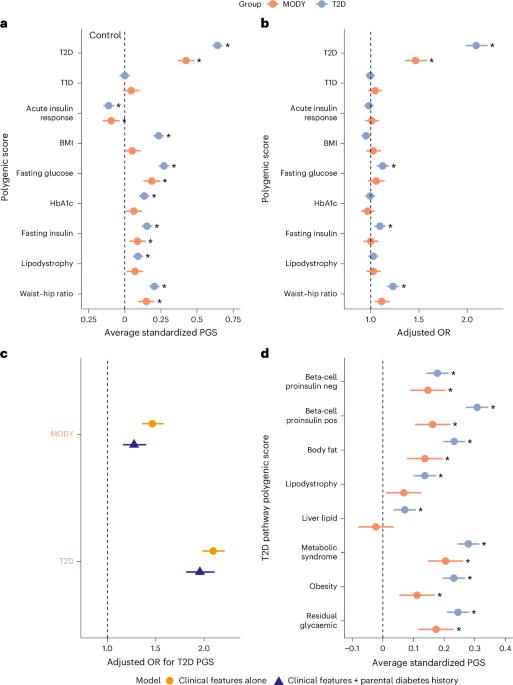
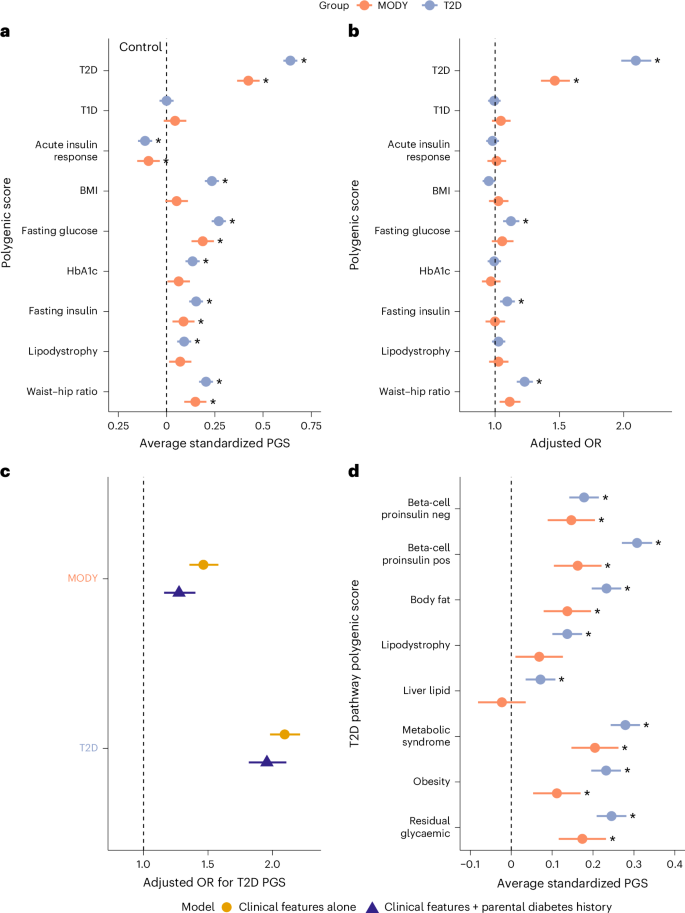
Young-onset monogenic disorders often show variable penetrance, yet the underlying causes remain poorly understood. Uncovering these influences could reveal new biological mechanisms and enhance risk prediction for monogenic diseases. Here we show that polygenic background substantially shapes the clinical presentation of maturity-onset diabetes of the young (MODY), a common monogenic form of diabetes that typically presents in adolescence or early adulthood. We find strong enrichment of type 2 diabetes (T2D) polygenic risk, but not type 1 diabetes risk, in genetically confirmed MODY cases (n = 1,462). This T2D polygenic burden, primarily through beta-cell dysfunction pathways, is strongly associated with earlier age of diagnosis and increased diabetes severity. Common genetic variants collectively account for 24% (P < 0.0001) of the phenotypic variability. Using a large population cohort (n = 424,553), we demonstrate that T2D polygenic burden substantially modifies diabetes onset in individuals with pathogenic variants, with diabetes risk ranging from 11% to 81%. Finally, we show that individuals with MODY-like phenotypes (n = 300) without a causal variant have elevated polygenic burden for T2D and related traits, representing potential polygenic phenocopies. These findings reveal substantial influence of common genetic variation in shaping the clinical presentation of early-onset monogenic disorders. Incorporating these may improve risk estimates for individuals carrying pathogenic variants. In clinical and population-based cohorts, a strong contribution of polygenic risk for type 2 diabetes (T2D) significantly modifies the onset and phenotypic variability of maturity-onset diabetes of the young (MODY). This polygenic T2D burden may also account for MODY-like individuals without identified monogenic causes.
常见的基因变异改变单基因糖尿病的疾病风险和临床表现
年轻发病的单基因疾病通常表现出不同的外显率,但潜在的原因仍然知之甚少。揭示这些影响可以揭示新的生物学机制,提高单基因疾病的风险预测。本研究表明,多基因背景在很大程度上决定了成熟型糖尿病(MODY)的临床表现,这是一种常见的单基因糖尿病,通常出现在青春期或成年早期。我们发现,在基因证实的MODY病例中,2型糖尿病(T2D)多基因风险显著增加,但1型糖尿病风险不明显(n = 1462)。这种T2D多基因负担,主要通过β细胞功能障碍途径,与早期诊断年龄和糖尿病严重程度增加密切相关。常见遗传变异合计占表型变异的24% (P < 0.0001)。通过一项大型人群队列研究(n = 424,553),研究人员证明,t2dm多基因负担显著改变了致病变异个体的糖尿病发病,其糖尿病风险范围为11%至81%。最后,我们发现,没有因果变异的mody样表型个体(n = 300)对T2D和相关性状的多基因负担升高,代表了潜在的多基因表型。这些发现揭示了共同遗传变异在塑造早发单基因疾病临床表现中的重要影响。将这些因素结合起来可以提高对携带致病变异个体的风险估计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


