HMGB1 contributes to pancreatic fibrosis by regulating TLR4-mediated autophagy and the NLRP3 inflammasome pathway in chronic pancreatitis
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
The characteristic pathological change in chronic pancreatitis (CP) is pancreatic fibrosis. In the early stages of CP development, injured acinar cells induce the infiltration of inflammatory cells, followed by pancreatic stellate cell (PSC) activation. Activated PSC induce the deposition of extracellular matrix (ECM) and promote the development of pancreatic fibrosis. High-mobility group Box 1 (HMGB1) is a damage-associated molecular pattern (DAMP) molecule. Although HMGB1 is implicated in several types of fibrotic diseases, its functional role and mechanism in pancreatic fibrosis in CP remain unknown. In this study, a dibutyltin dichloride (DBTC)-induced rat CP model was constructed in vivo. The results revealed that HMGB1 translocates from the nucleus of acinar cells to the cytoplasm and is subsequently released into the extracellular space, thereby promoting the progression of pancreatic fibrosis in CP. PSC was isolated and exposed to varying concentrations of HMGB1 in vitro. Resatorvid and si-TLR4 were applied to verify that the functions of HMGB1 were realized by combining with TLR4. 3-MA, si-Atg5 and MCC950 were used to determine the effect of HMGB1 on PSC activation through the regulation of autophagy and the NLRP3 inflammasome. These results indicated that exogenous administration of HMGB1 induces PSC activation and ECM deposition through its receptor TLR4. Mechanistically, the HMGB1/TLR4 axis promoted PSC activation by promoting autophagy–NLRP3 inflammasome activation. Our study highlights the profibrotic role of HMGB1 and provides a novel therapeutic target for the treatment of CP.
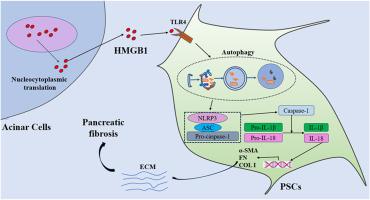
HMGB1通过调节慢性胰腺炎tlr4介导的自噬和NLRP3炎性体途径参与胰腺纤维化。
慢性胰腺炎(CP)的特征性病理改变是胰腺纤维化。在CP发展的早期,受损的腺泡细胞诱导炎症细胞浸润,随后是胰腺星状细胞(PSC)的激活。活化的PSC可诱导细胞外基质(ECM)沉积,促进胰腺纤维化的发生。高迁移率组框1 (HMGB1)是损伤相关分子模式(DAMP)分子。虽然HMGB1与几种类型的纤维化疾病有关,但其在CP患者胰腺纤维化中的功能作用和机制尚不清楚。本研究建立了二氯化二丁基锡(DBTC)诱导的大鼠CP体内模型。结果表明,HMGB1从腺泡细胞核转移到细胞质,随后释放到细胞外空间,从而促进CP胰腺纤维化的进展。分离PSC并在体外暴露于不同浓度的HMGB1。利用Resatorvid和si-TLR4验证HMGB1与TLR4结合后功能得以实现。利用3-MA、si-Atg5和MCC950检测HMGB1通过调节自噬和NLRP3炎性体对PSC活化的影响。这些结果表明,外源性HMGB1通过其受体TLR4诱导PSC活化和ECM沉积。机制上,HMGB1/TLR4轴通过促进自噬- nlrp3炎性体激活来促进PSC活化。我们的研究强调了HMGB1的促纤维化作用,为治疗CP提供了新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


