The crystal structure of a spherical vanadium complex encapsulating a nitrate anion
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-09-01
DOI:10.1107/S205698902500739X
引用次数: 0
Abstract
The crystal structure of a nitrate anion caged in spherical vanadium and oxygen structure surrounded by sodium hydroxy and water solvent molecules is reported.
The crystal structure of a nitrate anion caged in spherical vanadium and oxygen structure surrounded by sodium hydroxy and water solvent molecules, systematic name poly[[heptadecaaquatetradecaoxidononasodium][pentacosaaquanitratoundecaoxidopentadecavanadium]], H61NNa9O71V15 is reported. The complex crystallizes in the non-centrosymmetric Cc space group and exhibits many inter- and intramolecular hydrogen-bonding interactions. The complex contains VIV and VV centres, which are six-coordinate or octahedrally coordinated. The sodium atoms in this structure sit outside of the sphere with varying geometries and coordination numbers ranging from 5–8. The interactions between the sodium hydroxy sheet and spherical vanadium contributes to the packing of the molecules within the structure.
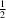
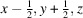
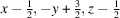
包覆硝酸阴离子的球形钒络合物的晶体结构。
报道了一种被球形钒笼住的硝酸阴离子的晶体结构和被氢氧钠和水溶剂分子包围的氧结构,系统名称为聚[[庚-十水-四-十氧化钠][五cosa-水-硝基十氧化五-十钒]],H61NNa9O71V15。该配合物在非中心对称的Cc空间群中结晶,并表现出许多分子间和分子内的氢键相互作用。该复合体包含VIV和VV中心,它们是六坐标或八面体协调的。这种结构中的钠原子以不同的几何形状和配位数在5-8之间分布在球体的外部。氢氧钠片与球形钒之间的相互作用有助于分子在结构内的堆积。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


