Synthesis and catalytic properties of copper(I) complexes with imino- and aminophosphine ligands
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
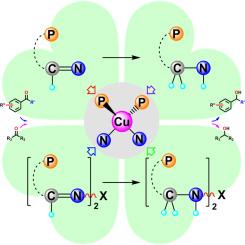
The iminophosphine ligands of o-Ph2PC6H4-CH=N-p-R-C6H4 (P^N-imine for short; R = OMe (1, L1 = MeO-ImP), Cl (2, L2 = Cl-ImP), Br (3, L3 = Br-ImP), COOH (4, L4 = COOH-ImP)) and bridged [o-Ph2PC6H4-CH=N]2-R (bis(P^N-imine) for short; R = -C2H4- (5, L5 = Et-bisImP), -C4H8- (6, L6 = Bu-bisImP), -C6H12- (7, L7 = Hex-bisImP)) were synthesized starting from o-Ph2PC6H4CHO with aryl/alkyl amines by condensation. In addition, amino derivatives of o-Ph2PC6H4-CH2-NH-p-R-C6H4 (P^NH-amine for short; R = Br (8, L8 = Br-AmP)) and bridged [o-Ph2PC6H4-CH2-NH]2-R (bis(P^NH-amine) for short; R = -C2H4- (9, L9 = Et-bisAmP), -C4H8- (10, L10 = Bu-bisAmP), -C6H12- (11, L11 = Hex-bisAmP)) were prepared by reduction of the imines (L1–7) with NaBH4. Treatment of imino- and aminophosphine ligands L1–11 with [Cu(MeCN)4]BF4 yielded the corresponding copper(I) complexes [Cu(L1–4)2]BF4 (1a-4a, L1 = MeO-ImP, L2 = Cl-ImP, L3 = Br-ImP, L4 = COOH-ImP), [Cu(L5–7)]BF4 (5a-7a, L5 = Et-bisImP, L6 = Bu-bisImP, L7 = Hex-bisImP), [Cu(L8)2]BF4 (8a, L8 = Br-AmP), and [Cu(L9–11)]BF4 (9a-11a, L9 = Et-bisAmP, L10 = Bu-bisAmP, L11 = Hex-bisAmP), while Ni(II) analogues [Ni(L5)](PF6)2 (5b, L5 = Et-bisImP) and [Ni(L9)Cl]PF6 (9b, L9 = Et-bisAmP) were prepared serving as comparators. The compositions and structures of the synthesized ligands and complexes were characterized with FT-IR and NMR spectroscopy and primarily single-crystal X-ray diffraction analyses. The resulting Cu(I) and Ni(II) complexes were evaluated as catalysts for the hydrosilylation of acetophenone under mild conditions. It is worth noting that the Cu(I) complex [Cu(L5)]BF4 (5a, L5 = Et-bisImP) achieved 93 %–99 % conversion in catalytic hydrosilylation of various aromatic and aliphatic ketones under mild conditions.
亚胺膦和氨基膦配体铜(I)配合物的合成及其催化性能
o-Ph2PC6H4-CH=N- P -R- c6h4(简称P^N-亚胺),R = OMe (1, L1 = MeO-ImP), Cl (2, L2 = Cl- imp), Br (3, L3 = Br- imp), COOH (4, L4 = COOH- imp))和桥接[o-Ph2PC6H4-CH=N]2-R (bis(P^N-亚胺))的亚膦配体;R = - c2h4 - (5, L5 = Et-bisImP), - c4h8 - (6, L6 = Bu-bisImP), - c6h12 - (7, L7 = hexx - bisimp)))由o-Ph2PC6H4CHO与芳基/烷基胺缩合合成。此外,o-Ph2PC6H4-CH2-NH- P -R- c6h4(简称P^NH-amine; R = Br (8, L8 = Br- amp))和桥接的[o-Ph2PC6H4-CH2-NH]2-R (bis(P^NH-amine))的氨基衍生物;用NaBH4还原亚胺(L1-7),制得R = - c2h4 - (9, L9 = Et-bisAmP)、- c4h8 - (10, L10 = Bu-bisAmP)、- c6h12 - (11, L11 = hexx - bisamp)。用[Cu(MeCN)4]BF4处理亚胺和氨基膦配体L1 -11得到相应的铜(I)配合物[Cu(L1 -4)2]BF4 (1a-4a, L1 = MeO-ImP, L2 = Cl- imp, L3 = Br-ImP, L4 = COOH-ImP), [Cu(L5 -7)]BF4 (5a-7a, L5 = Et-bisImP, L6 = Bu-bisImP, L7 = Hex-bisImP), [Cu(L8)2]BF4 (8a, L8 = Br-AmP)和[Cu(L9 -11)]BF4 (9a-11a, L9 = Et-bisAmP, L10 = Bu-bisAmP, L11 = Hex-bisAmP),而Ni(II)类似物[Ni(L5)](PF6)2 (5b, L5 = Et-bisImP)和[Ni(L9)Cl]PF6 (9b,L9 = Et-bisAmP)作为比较物。合成的配体和配合物的组成和结构通过红外光谱和核磁共振光谱以及主要的单晶x射线衍射分析进行了表征。在温和条件下评价了Cu(I)和Ni(II)配合物对苯乙酮硅氢化反应的催化作用。值得注意的是,Cu(I)配合物[Cu(L5)]BF4 (5a, L5 = Et-bisImP)在温和条件下催化各种芳酮和脂肪酮的硅氢化反应中转化率达到93% - 99%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


