Synthesis, structural insights, antimicrobial potency, and theoretical studies of Cu(II), Ni(II), and Cd(II) complexes derived from Coumarin-based 1,2,4-Triazine
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A novel hydrazone ligand, 4-{[−1-(4-hydroxycoumarin-3-yl)ethylidene]amino}-6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one (CEAT), was synthesized via condensation of 3-acetyl-4-hydroxycoumarin with 4-amino-6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Coordination of CEAT with Ni2+, Cu2+, and Cd2+ ions afforded M(CEAT) complexes in a 1:1 stoichiometric ratio. The current compounds were characterized by elemental analysis, TGA, magnetic susceptibility, molar conductivity, IR, 1H and 13C NMR, mass spectrometry, UV–Vis, ESR, and XRD. Spectral data confirmed CEAT as a monobasic ONS tridentate ligand, coordinating via azomethine nitrogen, phenolic oxygen, and thione sulfur atoms, forming a tetrahedral geometry for Cu(CEAT) and octahedral geometries for Ni(CEAT) and Cd(CEAT) complexes. Also, the overall shape and nature of the ESR signal are consistent with a distorted tetrahedral geometry around the copper center. The molar conductivity data indicate that Cu(CEAT) and Ni(CEAT) complexes are neutral, whereas Cd(CEAT) falls within the characteristic range for a 1:1 electrolyte. The XRD analysis confirmed the crystalline nature of the present complexes with crystallite sizes of 12–21 nm. Antimicrobial evaluation showed that complexation markedly enhanced bioactivity compared to the free ligand, where Ni(CEAT) exhibiting the strongest antibacterial effect against Escherichia coli (MIC = 19.2 μg/mL) and notable antifungal potency against Candida albicans (MIC = 15.4 μg/mL). Density Functional Theory (DFT) calculations were performed to optimize the molecular geometries of the CEAT ligand and its metal complexes, alongside the evaluation of key quantum chemical descriptors. Moreover, the computed first hyperpolarizability values of the synthesized compounds are better than those of urea, suggesting their promising potential for nonlinear optical (NLO) applications. Comparative analysis between theoretical predictions and experimental antimicrobial results provided mechanistic insight into enhanced bioactivity. In silico ADMET and toxicity profiling further indicated that the CEAT ligand and its complexes possess favorable pharmacokinetic attributes, including suitable oral bioavailability, low predicted systemic toxicity, and high metabolic compatibility. Molecular docking against E. coli 1HNJ protein demonstrated strong and stable binding, where Ni(CEAT) showing the highest affinity (−10.30 kcal/mol), correlating with its superior in vitro antimicrobial performance.
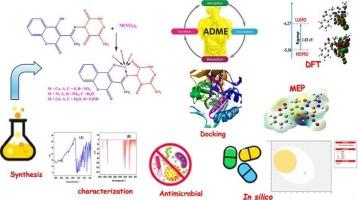
以香豆素为基础的1,2,4-三嗪衍生的Cu(II), Ni(II)和Cd(II)配合物的合成、结构见解、抗菌效力和理论研究
以3-乙酰基-4-羟基香豆素与4-氨基-6-甲基-3-甲基-3-硫氧-3,4-二氢-3,4-二氢-1,2,4-三嗪-5(2H)- 1缩合为原料,合成了新型腙配体4-{[−1-(4-羟基香豆素-3-酰基)乙基]氨基}-6-甲基-3-硫氧-3,4-二氢-1,2,4-三嗪-5(2H)- 1 (CEAT)。CEAT与Ni2+、Cu2+和Cd2+离子配位,形成M(CEAT)配合物,化学计量比为1:1。通过元素分析、TGA、磁化率、摩尔电导率、IR、1H和13C NMR、质谱、UV-Vis、ESR和XRD等手段对化合物进行了表征。光谱数据证实,CEAT是一种单碱性的ONS三叉体配体,通过亚甲基氮、酚氧和硫原子配位,形成Cu(CEAT)的四面体结构,Ni(CEAT)和Cd(CEAT)的八面体结构。此外,ESR信号的整体形状和性质与铜中心周围扭曲的四面体几何形状一致。摩尔电导率数据表明,Cu(CEAT)和Ni(CEAT)配合物是中性的,而Cd(CEAT)则处于1:1电解质的特征范围内。XRD分析证实了该配合物的结晶性质,晶粒尺寸为12 ~ 21 nm。抗菌评价表明,与游离配体相比,配合物的生物活性明显增强,其中Ni(CEAT)对大肠杆菌(MIC = 19.2 μg/mL)的抑菌效果最强,对白色念珠菌(MIC = 15.4 μg/mL)的抑菌效果显著。密度泛函理论(DFT)计算优化了CEAT配体及其金属配合物的分子几何结构,并对关键量子化学描述符进行了评估。此外,合成的化合物的第一超极化率值比尿素的高,这表明它们在非线性光学(NLO)应用方面具有广阔的潜力。理论预测和实验抗菌结果之间的比较分析提供了增强生物活性的机制见解。ADMET和毒性分析进一步表明,CEAT配体及其复合物具有良好的药代动力学特性,包括适宜的口服生物利用度、低预测全身毒性和高代谢相容性。与大肠杆菌1HNJ蛋白的分子对接表现出较强且稳定的结合,其中Ni(CEAT)的亲和力最高(−10.30 kcal/mol),具有较好的体外抗菌性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


