Prim-O-glucosylcimifugin attenuates intestinal fibrosis by modulating TGF-β/MAPK signaling and ECM remodeling
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Background
Intestinal fibrosis is a severe and progressive complication of inflammatory bowel disease (IBD), particularly Crohn's disease (CD), for which no effective anti-fibrotic therapies currently exist.
Purpose
This study aimed to investigate the anti-fibrotic efficacy and underlying mechanisms of Prim-O-glucosylcimifugin (POG), a natural chromone derivative, in TGF-β1-stimulated human intestinal fibroblasts.
Methods
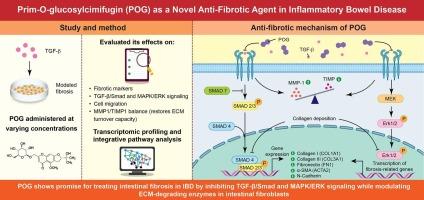
Fibrosis was modeled in human intestinal fibroblast cell lines (CCD-18Co) and human primary intestinal myofibroblasts (HIMF) using TGF-β1. POG was administered at varying concentrations, and its effects on fibrotic marker expression, MMP1 activity, and cell migration were evaluated using qPCR, western blotting, immunofluorescence, and wound healing assays. Transcriptomic profiling and integrative pathway analysis were used to identify target signaling cascades.
Results
POG significantly attenuated TGF-β-induced myofibroblast activation, reducing α-SMA, fibronectin, collagen I/III, and N-cadherin levels. Mechanistically, POG suppressed both canonical TGF-β/Smad and non-canonical MAPK/ERK signaling and enhanced extracellular matrix (ECM) turnover by upregulating MMP1 while downregulating TIMP1. Transcriptomic analysis corroborated the involvement of ECM remodeling and ERK pathway inhibition. Importantly, POG exhibited no cytotoxicity in intestinal epithelial cells.
Conclusion
POG demonstrates anti-fibrotic potential in intestinal fibroblasts via dual inhibition of the TGF-β/Smad and MAPK/ERK pathways and selective modulation of ECM-degrading enzymes. These findings highlight POG as a promising therapeutic candidate for the treatment of intestinal fibrosis in IBD.
prim - o - glucosylcimifgin通过调节TGF-β/MAPK信号和ECM重塑来减轻肠纤维化
肠道纤维化是炎症性肠病(IBD)的一种严重的进行性并发症,尤其是克罗恩病(CD),目前尚无有效的抗纤维化治疗方法。目的探讨天然色素衍生物Prim-O-glucosylcimifugin (POG)对TGF-β1刺激的人肠成纤维细胞的抗纤维化作用及其机制。方法采用TGF-β1诱导人肠成纤维细胞(CCD-18Co)和人原代肠肌成纤维细胞(HIMF)纤维化。使用不同浓度的POG,通过qPCR、western blotting、免疫荧光和伤口愈合试验评估其对纤维化标志物表达、MMP1活性和细胞迁移的影响。转录组学分析和综合途径分析用于识别目标信号级联。结果spog显著减弱TGF-β诱导的肌成纤维细胞活化,降低α-SMA、纤维连接蛋白、胶原I/III和n -钙粘蛋白水平。在机制上,POG通过上调MMP1和下调TIMP1抑制典型TGF-β/Smad和非典型MAPK/ERK信号,并增强细胞外基质(ECM)的周转。转录组学分析证实了ECM重塑和ERK通路抑制的参与。重要的是,POG对肠上皮细胞没有细胞毒性。结论pog通过双重抑制TGF-β/Smad和MAPK/ERK通路以及选择性调节ecm降解酶,在肠成纤维细胞中具有抗纤维化潜能。这些发现强调了POG作为治疗IBD肠道纤维化的有希望的治疗候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


