Targeting FSP1 to induce ferroptosis in chromophobe renal cell carcinoma
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
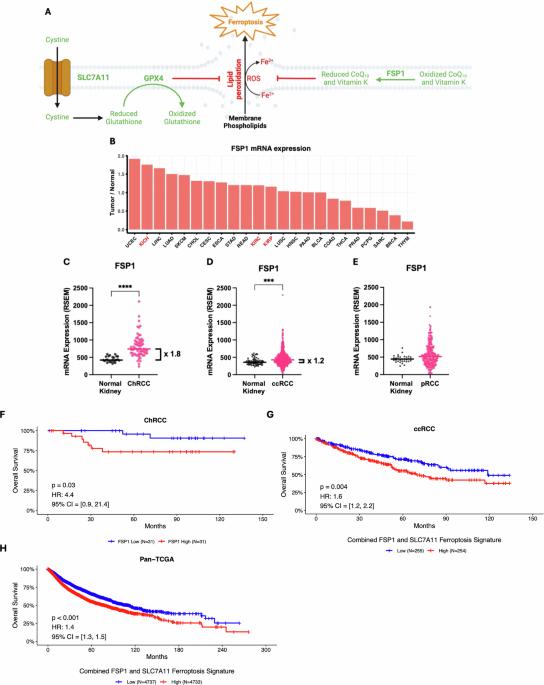
There are no proven therapies for metastatic or unresectable Chromophobe Renal Cell Carcinoma (ChRCC). ChRCC is characterized by high glutathione levels and hypersensitivity to ferroptosis, an iron-dependent form of cell death characterized by peroxidation of polyunsaturated fatty acids. The underlying mechanisms leading to ferroptosis hypersensitivity are unknown. Ferroptosis suppressor protein (FSP1) is a glutathione-independent suppressor of ferroptosis whose role in ChRCC is unexplored. In The Cancer Genomic Atlas (TCGA), we find that ChRCC exhibits the second highest upregulation of FSP1 relative to healthy organ out of all cancers, and that higher FSP1 expression correlates with poorer patient outcomes. We also define a ferroptosis signature combining FSP1 and Solute Carrier Family 7 Member 11 (SLC7A11) that predicts patient survival across all TCGA tumor types. Data queried from the Dependency Map and the Cancer Target Discovery and Development indicate that high FSP1 expression correlates with resistance to cell death induced by disruption of glutathione homeostasis via inhibition of glutathione peroxidase 4 (GPX4) or SLC7A11. Studies using ChRCC cell lines in vitro reveal that genetic inhibition of GPX4 or FSP1 individually does not induce substantial cell death, while inhibition of both results in near-complete loss of viability. Consistent with these genetic data, combining pharmacologic inhibition of GPX4 or SLC7A11 with inhibition of FSP1 demonstrates synergistic loss of viability. Strikingly, inhibition of FSP1 alone in vivo is sufficient to decrease ChRCC tumor growth by 69%, consistent with recent studies in lung and colorectal cancer showing similar effects. Taken together, these data establish FSP1 as targetable vulnerability in ChRCC.
靶向FSP1诱导嗜色肾细胞癌铁下垂。
对于转移性或不可切除的嫌色性肾细胞癌(ChRCC),尚无证实的治疗方法。ChRCC的特点是高谷胱甘肽水平和对铁死亡过敏,铁死亡是一种铁依赖性的细胞死亡形式,以多不饱和脂肪酸的过氧化为特征。导致铁下垂过敏的潜在机制尚不清楚。铁下垂抑制蛋白(FSP1)是一种不依赖谷胱甘肽的铁下垂抑制蛋白,其在ChRCC中的作用尚不清楚。在癌症基因组图谱(TCGA)中,我们发现在所有癌症中,相对于健康器官,ChRCC表现出第二高的FSP1上调,并且较高的FSP1表达与较差的患者预后相关。我们还定义了结合FSP1和溶质载体家族7成员11 (SLC7A11)的铁下垂特征,预测所有TCGA肿瘤类型的患者生存。依赖性图谱和Cancer Target Discovery and Development的数据显示,FSP1的高表达与通过抑制谷胱甘肽过氧化物酶4 (gtathione peroxidase 4, GPX4)或SLC7A11破坏谷胱甘肽内稳态诱导的细胞死亡的抗性相关。体外对ChRCC细胞系的研究表明,GPX4或FSP1单独的基因抑制不会诱导细胞大量死亡,而两者的抑制会导致细胞活力几乎完全丧失。与这些遗传数据一致的是,GPX4或SLC7A11的药物抑制与FSP1的抑制联合显示出协同丧失活力。引人注目的是,体内单独抑制FSP1足以使ChRCC肿瘤生长减少69%,这与最近在肺癌和结直肠癌中的研究显示的相似效果一致。综上所述,这些数据确立了FSP1是ChRCC中可攻击的漏洞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


