Protective role of Bre1 in mitochondrial function and energy metabolism in Drosophila models of Parkinson's disease
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
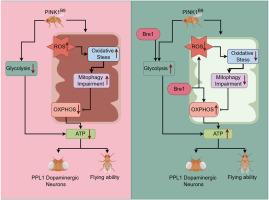
The second most common cause of autosomal recessive early-onset Parkinson's disease (PD) can be attributed to mutations in the PINK1 gene, malfunction of the mitochondria is the key pathological mechanism. Bre1 encodes an E3 ubiquitin ligase, with the discovery of Bre1's role in repairing mitochondrial damage, further investigation into its implications for PD is warranted.
Methods
We used the PINK1B9 drosophila melanogaster as the PD model. The effects of Bre1 on PD phenotypes were evaluated based on the morphology of the wings and dorsal region, as well as flight ability. Immunostaining of dopaminergic neurons was used to examine neurodegeneration. Transcriptomes were used to detect the pathway directly involved. Mitochondrial structure and function were observed using electron microscopy, ATP detection, and an oxygen consumption assay. The detection of SOD activity and ROS were used to explicit the effects of Bre1 on oxidative stress. To identify the effects of Bre1 on glycolysis and tricarboxylic acid (TCA) cycle, we performed Western Blot and RT-PCR.
Results
We discovered that Bre1 overexpression significantly improved the phenotype of PD flies and protected their dopaminergic neurons from degeneration. More significantly, we observed that the overexpression of Bre1 markedly enhanced the respiratory capacity of mitochondrial Complex I and Complex II, elevated ATP levels, reduced ROS levels, and improved mitochondrial structural integrity. The Western Blot results demonstrate a significant increase in the critical glycolysis enzymes, Pfk and Pyk proteins. Moreover, qRT-PCR results showed a remarkably upregulation in the transcriptional level of OGDH, a critical rate-limiting enzyme in the TCA cycle. Therefore, our study suggests that Bre1 improves the phenotypes of PD model flies by attenuating mitochondrial damage and enhancing energy metabolism, offering a potential drug target for ameliorating the symptoms of PINK1 mutant autosomal recessive PD patients.
Bre1在帕金森病果蝇模型线粒体功能和能量代谢中的保护作用
背景:常染色体隐性遗传早发性帕金森病(PD)的第二大常见病因可归因于PINK1基因突变,线粒体功能障碍是其关键病理机制。Bre1编码E3泛素连接酶,随着Bre1在修复线粒体损伤中的作用的发现,进一步研究其对帕金森病的影响是有必要的。方法:以PINK1B9黑腹果蝇为PD模型。Bre1对PD表型的影响基于翅膀和背部区域的形态以及飞行能力进行评估。多巴胺能神经元免疫染色检测神经变性。转录组被用来检测直接参与的途径。用电镜、ATP检测和耗氧量测定观察线粒体结构和功能。通过检测SOD活性和ROS来明确Bre1对氧化应激的影响。为了确定Bre1对糖酵解和三羧酸(TCA)循环的影响,我们进行了Western Blot和RT-PCR。结果:我们发现Bre1过表达可显著改善PD蝇的表型,保护其多巴胺能神经元不变性。更重要的是,我们观察到Bre1的过表达显著增强了线粒体复合体I和复合体II的呼吸能力,提高了ATP水平,降低了ROS水平,改善了线粒体结构完整性。Western Blot结果显示,关键的糖酵解酶Pfk和Pyk蛋白显著增加。此外,qRT-PCR结果显示,TCA循环中关键限速酶OGDH的转录水平显著上调。因此,我们的研究表明,Bre1通过减轻线粒体损伤和增强能量代谢来改善PD模型果蝇的表型,为改善PINK1突变型常染色体隐性PD患者的症状提供了潜在的药物靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


