LncRNA BASP1-AS1 drives PCBP2 K115 lactylation to suppress ferroptosis and confer oxaliplatin resistance in gastric cancer
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In oxaliplatin-resistant gastric cancer (GC), multi-omics profiling combined with organoid libraries reveals altered metabolic pathways associated with chemoresistance. We identify a novel lactylation modification at K115 of Poly(RC)-binding protein 2 (PCBP2K115la), which confers functional oxaliplatin resistance. Mechanistic studies demonstrate that the long non-coding RNA BASP1-AS1 assembles a complex containing Unc-51 Like Autophagy Activating Kinase 1 (ULK1) and lactate dehydrogenase A (LDHA), thereby activating LDHA enzymatic activity to increase lactate production. Elevated lactate triggers PCBP2K115la modification, disrupting PCBP2-ARIH2 interaction to inhibit ubiquitin-dependent degradation and stabilize PCBP2. Concurrently, BASP1-AS1-mediated histone H3K14 lactylation transcriptionally upregulates both LDHA and PCBP2, generating a self-amplifying metabolic-epigenetic circuit. This axis critically suppresses ferroptosis and maintains chemoresistance, providing actionable targets for overcoming oxaliplatin resistance in GC.
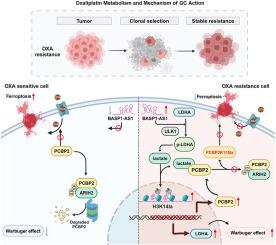
LncRNA BASP1-AS1驱动PCBP2 K115乳酸化抑制胃癌中铁下沉并赋予奥沙利铂耐药
在奥沙利铂耐药胃癌(GC)中,结合类器官文库的多组学分析揭示了与化疗耐药相关的代谢途径改变。我们在Poly(RC)结合蛋白2 (PCBP2K115la)的K115位点发现了一种新的乳酸化修饰,该修饰赋予了功能性奥沙利铂耐药性。机制研究表明,长链非编码RNA BASP1-AS1组装了含有Unc-51样自噬激活激酶1 (ULK1)和乳酸脱氢酶a (LDHA)的复合体,从而激活LDHA酶活性来增加乳酸的产生。乳酸升高触发PCBP2K115la修饰,破坏PCBP2- arih2相互作用,抑制泛素依赖性降解,稳定PCBP2。同时,basp1 - as1介导的组蛋白H3K14乳酸化转录上调LDHA和PCBP2,产生一个自我放大的代谢-表观遗传回路。该轴严重抑制铁下沉并维持化学耐药,为克服GC中的奥沙利铂耐药提供了可行的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


