Exendin-4 Prevents oxLDL-Induced upregulation of TREM2 and attenuates foam cell formation and inflammation in Macrophages
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Atherosclerosis (AS), a chronic inflammatory disease and a leading cause of cardiovascular morbidity and mortality. Macrophage-mediated lipid uptake and inflammation are central to plaque formation. TREM2, an immunoreceptor expressed in macrophages, has been reported to regulate lipid metabolism and inflammation, yet its role in atherosclerosis remains controversial. Exendin-4, a GLP-1 receptor agonist with its known cardiovascular protective effects, may influence immune signaling beyond glycemic control. However, whether the effect of Exendin-4 mitigating AS and the relations with TREM2 and its downstream JAK2/STAT3 pathway are unknown. We aimed to investigate the role of the Exendin-4-TREM2-JAK2/STAT3 axis in foam cell formation and inflammation during AS progression. OxLDL was used to stimulate THP-1-derived macrophages, and ApoE−/− mice fed a high-fat diet were used to construct an in vivo AS model. TREM2 expression was manipulated using lentiviral vectors, and the role of JAK2 signaling was assessed with a specific inhibitor. We evaluated the effects of Exendin-4 on TREM2 expression, foam cell formation and inflammation. We found that lipid stimulation increased TREM2 expression and activated the JAK2/STAT3 pathway in macrophages, leading to enhanced foam cell formation and pro-inflammatory cytokine production. Also, TREM2 overexpression caused increasing levels of inflammatory cytokines and foam cells. Exendin-4 could alleviate AS progression under the anti-inflammatory and anti-foaming effects, with reducing TREM2 expressions. However, the inflammation and foam cell formation with JAK2/STAT3 pathway activation caused by TREM2 overexpression cannot be reversed by Exendin-4. All our findings indicate that Exendin-4 suppress foam cell formation and inflammatory responses, with inhibiting macrophage TREM2 expression up-regulation.
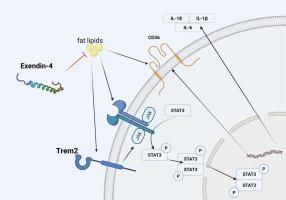
Exendin-4阻止氧化低密度脂蛋白诱导的TREM2上调,减轻巨噬细胞泡沫细胞形成和炎症。
动脉粥样硬化(AS)是一种慢性炎症性疾病,也是心血管疾病发病率和死亡率的主要原因。巨噬细胞介导的脂质摄取和炎症是斑块形成的核心。据报道,巨噬细胞中表达的免疫受体TREM2调节脂质代谢和炎症,但其在动脉粥样硬化中的作用仍存在争议。Exendin-4是一种已知具有心血管保护作用的GLP-1受体激动剂,可能影响血糖控制以外的免疫信号。然而,Exendin-4是否有缓解AS的作用以及与TREM2及其下游JAK2/STAT3通路的关系尚不清楚。我们的目的是研究Exendin-4-TREM2-JAK2/STAT3轴在AS进展过程中泡沫细胞形成和炎症中的作用。用OxLDL刺激thp -1来源的巨噬细胞,并用高脂饮食喂养ApoE-/-小鼠构建体内AS模型。使用慢病毒载体操纵TREM2表达,并使用特定抑制剂评估JAK2信号传导的作用。我们评估了Exendin-4对TREM2表达、泡沫细胞形成和炎症的影响。我们发现脂质刺激增加巨噬细胞TREM2表达并激活JAK2/STAT3通路,导致泡沫细胞形成和促炎细胞因子产生增强。此外,TREM2过表达导致炎症细胞因子和泡沫细胞水平升高。Exendin-4在抗炎和消泡作用下可减缓AS进展,降低TREM2的表达。然而,TREM2过表达引起的炎症和泡沫细胞形成与JAK2/STAT3通路激活不能被Exendin-4逆转。我们的研究结果表明,Exendin-4抑制泡沫细胞形成和炎症反应,抑制巨噬细胞TREM2表达上调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


