Disordered Ferlin C2A–C2B Linkers Bind Membranes and Encode Small Linear Motifs
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ferlins are vesicle trafficking proteins composed of folded C2 domains conjugated by linkers which are largely disordered. Although a role for the C2 domains as calcium sensors has been established it remains unclear whether the linkers function beyond acting as passive spacers. We examined the C2A–C2B linker sequences of vertebrate ferlins and found both putative short linear motifs (SLiMs) as well as membrane binding sequences for members of the protein family. Specifically, for otoferlin we identified an arginine-rich region proximal to an AP2 binding dileucine motif which interacts with negatively charged lipid membranes. Further, the linker region dominated the liposome binding properties of a larger recombinant C2A–C2B, two-C2 domain segment of otoferlin, suggesting a dominant role in mediating the membrane binding property of the N-terminus. We also found that alternative splicing of the otoferlin C2A–C2B linker adds an additional membrane binding segment and alters the affinity of membrane binding. Like otoferlin, a recombinant dysferlin linker interacted with liposomes. However, dysferlin encodes for SLiMs not detected in the otoferlin linker and interacted with both SH3- and WW- domain proteins as determined using fluorescence spectroscopy. We conclude that the C2A–C2B linker of vertebrate ferlins serves as a signaling platform by recruiting SLiM-binding partners. Membrane binding “hotspots” encoded in a subset of linkers including otoferlin may serve to localize protein complexes proximal to the cell membrane for activity.
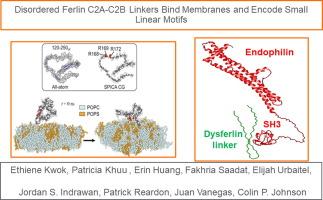
紊乱的Ferlin C2A-C2B连接体结合膜并编码小线性基序。
Ferlins是由折叠的C2结构域组成的囊泡运输蛋白,由大量无序的连接物偶联而成。虽然C2结构域作为钙传感器的作用已经确立,但连接体是否具有被动间隔器以外的功能仍不清楚。我们研究了脊椎动物ferlins的C2A-C2B连接序列,发现了该蛋白家族成员的推定短线性基序(slms)和膜结合序列。具体来说,对于otoferlin,我们发现了一个富含精氨酸的区域,该区域靠近AP2结合二亮氨酸基序,与带负电荷的脂质膜相互作用。此外,连接子区域主导了较大的重组C2A-C2B, otoferlin的2 - c2结构域片段的脂质体结合特性,表明在介导n端膜结合特性中起主导作用。我们还发现,otoferlin C2A-C2B连接体的选择性剪接增加了一个额外的膜结合片段,并改变了膜结合的亲和力。与otoferlin一样,重组异铁蛋白连接体与脂质体相互作用。然而,dysferlin编码在otoferlin连接体中未检测到的slms,并通过荧光光谱测定与SH3-和WW-结构域蛋白相互作用。我们得出结论,脊椎动物ferlins的C2A-C2B连接体通过招募SLiM-binding伴侣作为信号平台。在包括otoferlin在内的连接子子集中编码的膜结合“热点”可能用于定位靠近细胞膜的蛋白复合物的活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


