Molecular insights into the role of ferroptosis in cardiorenal cross-talk: Mechanisms and future directions
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Cardiorenal syndrome (CRS) is a bidirectional relationship shared between the heart and kidneys, both in physiological and pathophysiological perspectives. The metabolic, hemodynamic, and neurohormonal alterations between the heart and kidneys drive this dual-organ damage and are responsible for one of the highest medical concerns around the globe. From a pathophysiological perspective, activation of the renin-angiotensin system, persistent inflammation, oxidative stress, and reactive fibrosis are accountable for the damage to the heart and kidneys. The review focuses on ferroptosis, which is an iron-dependent lipid peroxidation of the plasma membrane that directs the cell towards cell death. The iron-catalyzed lipid peroxides (LOOH), redox imbalance, inactivation of protective machinery systems such as system Xc, glutathione peroxidase (GPX4), increased iron intake by divalent metal transporter 1 (DMT1) and transferrin receptor 1(TFR1), and ferritinophagy promote cellular lipid peroxidation, the fenton reaction, and intracellular Fe+2 overload that disrupts homeostasis, and the cells are directed towards ferroptotic cell death. Recently, ferroptotic cell death has been described in a multitude of kidney and cardiac disorders, including acute and chronic kidney diseases, myocardial infarction, heart failure, and so on. This review summarizes recent developments in the context of ferroptosis and its involvement in CRS. The molecular pathways and mechanisms, and how modulating the same could be beneficial for dual-organ protection in the heart and kidneys, are discussed.
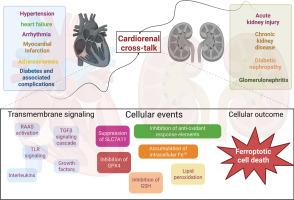
铁下垂在心肾串扰中的分子作用:机制和未来方向。
心肾综合征(CRS)是心脏和肾脏在生理和病理生理方面的双向关系。心脏和肾脏之间的代谢、血液动力学和神经激素的改变驱动了这种双器官损伤,是全球最受关注的医学问题之一。从病理生理学的角度来看,肾素-血管紧张素系统的激活、持续的炎症、氧化应激和反应性纤维化是造成心脏和肾脏损伤的原因。铁死亡是一种依赖铁的质膜脂质过氧化,导致细胞死亡。铁催化的脂质过氧化物(LOOH)、氧化还原失衡、保护机制系统(如系统Xc、谷胱甘肽过氧化物酶(GPX4))失活、DMT1和转铁蛋白受体1(TFR1)铁摄入量增加以及铁蛋白自噬促进细胞脂质过氧化、芬顿反应和细胞内Fe+2超载,破坏体内平衡,细胞被导向铁致细胞死亡。近年来,许多肾脏和心脏疾病,包括急性和慢性肾脏疾病、心肌梗死、心力衰竭等,都描述了嗜铁细胞死亡。本文综述了近年来关于铁下垂及其在CRS中的作用的研究进展。讨论了分子途径和机制,以及如何调节它们对心脏和肾脏的双器官保护有益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


