NOTCH signaling orchestrates the inflammatory-fibrotic continuum of macrophages in renal allograft rejection
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Background
Chronic rejection is a major cause of long-term kidney allograft failure, characterized by persistent inflammation and progressive fibrosis. Macrophages are central mediators of this process, but their phenotypic heterogeneity and regulatory mechanisms in chronic rejection remain incompletely understood.
Methods
We performed single-cell transcriptomic analysis on renal allograft biopsies from patients with different types of rejection and on a time-course rat model of chronic rejection. Macrophage subsets were identified through transcriptional profiling and Pseudotime trajectory analysis. Ligand–receptor analysis defined upstream intercellular communication, while in vitro assays using THP-1 macrophages evaluated responses to Jagged1 stimulation under polarizing conditions.
Results
A distinct TGFB+CD86+ macrophage subset exhibiting both pro-inflammatory and pro-fibrotic features was identified. This population, enriched in mixed rejection, occupied an intermediate position along the inferred macrophage trajectory and displayed dual ontogeny. It received Jagged1–NOTCH2 signals from mesenchymal-transitioned tubular epithelial cells and inflammatory inputs from infiltrating T cells. In vitro, co-stimulation with soluble Jagged1 under M1-polarizing conditions induced a similar hybrid phenotype. In the rat model, a phenotypically comparable subset, provisionally termed M2b, appeared early post-transplantation and was later replaced by M2a macrophages as fibrosis progressed. Ligand–receptor analysis confirmed conserved Jagged1–NOTCH2 signaling regulatory axis in vivo.
Conclusion
In summary, we identify a transitional TGFB+CD86+ macrophage population governed by JAG1–NOTCH2 signaling, bridging immune activation and fibrotic remodeling. Modulating this pathway may offer a therapeutic approach to reshape macrophage differentiation and mitigate chronic rejection.
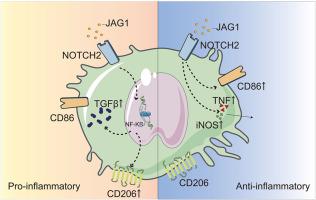
NOTCH信号在同种异体肾移植排斥反应中协调巨噬细胞的炎症-纤维化连续体。
背景:慢性排斥反应是长期同种异体肾移植失败的主要原因,其特征是持续的炎症和进行性纤维化。巨噬细胞是这一过程的中心介质,但其在慢性排斥反应中的表型异质性和调节机制仍不完全清楚。方法:我们对不同类型排斥反应患者的肾移植活检组织和慢性排斥反应大鼠模型进行了单细胞转录组学分析。通过转录谱分析和伪时间轨迹分析确定巨噬细胞亚群。配体受体分析定义了上游细胞间通讯,而THP-1巨噬细胞在体外实验中评估了极化条件下对Jagged1刺激的反应。结果:发现了一个独特的TGFB+CD86+巨噬细胞亚群,显示出促炎和促纤维化的特征。这个群体富含混合排斥反应,在推断的巨噬细胞轨迹中占据中间位置,并表现出双重个体发生。它接受来自间质转移的小管上皮细胞的Jagged1-NOTCH2信号和浸润性T细胞的炎症输入。在体外,与可溶性Jagged1在m1极化条件下共刺激诱导了类似的杂交表型。在大鼠模型中,表型相似的亚群,暂时称为M2b,在移植后早期出现,后来随着纤维化的进展被M2a巨噬细胞所取代。配体-受体分析证实Jagged1-NOTCH2信号调控轴在体内是保守的。结论:总之,我们确定了一个过渡性TGFB+CD86+巨噬细胞群体,由JAG1-NOTCH2信号、桥接免疫激活和纤维化重塑控制。调节这一途径可能为重塑巨噬细胞分化和减轻慢性排斥提供治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


