Overcoming standard-of-care resistance in glioblastoma using nanoparticle-based drug delivery targeting the autophagy pathway
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Glioblastoma (GBM) is the most aggressive and lethal primary brain tumor in adults, characterized by rapid growth, diffuse infiltration, and a dismal prognosis. Despite aggressive treatment involving maximal surgical resection followed by radiotherapy and temozolomide (TMZ) chemotherapy, therapeutic outcomes remain poor due to intrinsic and acquired resistance. Autophagy, a catabolic process that degrades damaged cellular components, plays a critical role in this resistance by enabling tumor cells to survive under metabolic, hypoxic, and therapeutic stress conditions. Notably, modulation of autophagy has emerged as a promising avenue to overcome drug resistance. Recent advances in nanomedicine offer innovative strategies to enhance drug delivery and therapeutic efficacy. Nanoparticle-based drug delivery systems (NDDS) improve the bioavailability of drug molecules, facilitate blood–brain barrier (BBB) penetration, and enable targeted delivery to tumor tissues. This review explores the synergistic potential of integrating NDDS with autophagy-targeting strategies to treat GBM. Various nanoparticle platforms-including liposomes, dendrimers, polymeric nanoparticles, and lipid-based carriers-are highlighted for their ability to modulate autophagy and deliver anti-cancer agents effectively. Furthermore, we discuss the dual role of autophagy in GBM progression and the importance of context- and time-specific modulation. Thus, combining autophagy inhibitors or modulators with nanoparticle-based systems and standard therapies holds promise as a novel therapeutic strategy to counteract resistance and improve patient survival in GBM.
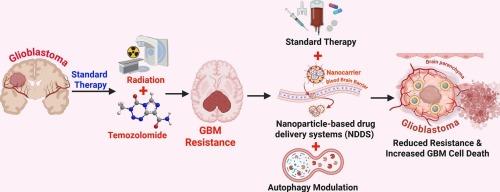
利用靶向自噬途径的纳米颗粒为基础的药物递送克服胶质母细胞瘤的标准治疗耐药性。
胶质母细胞瘤(GBM)是成人最具侵袭性和致死性的原发性脑肿瘤,其特点是生长迅速,弥漫性浸润,预后差。尽管积极的治疗包括最大手术切除,放疗和替莫唑胺(TMZ)化疗,但由于内在和获得性耐药,治疗结果仍然很差。自噬是一种降解受损细胞成分的分解代谢过程,通过使肿瘤细胞在代谢、缺氧和治疗应激条件下存活,在这种耐药性中起着关键作用。值得注意的是,自噬调节已成为克服耐药性的有希望的途径。纳米医学的最新进展为提高药物传递和治疗效果提供了创新的策略。基于纳米颗粒的药物递送系统(NDDS)提高了药物分子的生物利用度,促进了血脑屏障(BBB)的穿透,并使靶向递送到肿瘤组织成为可能。这篇综述探讨了将NDDS与自噬靶向治疗GBM的协同潜力。各种纳米粒子平台——包括脂质体、树状大分子、聚合物纳米粒子和基于脂质的载体——因其调节自噬和有效递送抗癌药物的能力而受到重视。此外,我们讨论了自噬在GBM进展中的双重作用以及环境和时间特异性调节的重要性。因此,将自噬抑制剂或调节剂与基于纳米颗粒的系统和标准疗法相结合,有望成为一种新的治疗策略,以抵消耐药性并提高GBM患者的生存率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


