Rational design and translational advancement of phospholipid-based nanocarriers for targeted cancer therapy
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
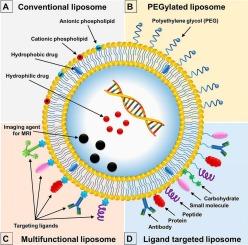
Phospholipid-derived nanocarriers represent a versatile and chemically customizable class of drug delivery systems that self-assemble into bilayered vesicles due to their intrinsic amphiphilicity. These systems can encapsulate both hydrophilic and hydrophobic drugs through non-covalent interactions and manipulation of lipid phase behavior. This review examines the molecular and supramolecular principles underlying the formation, stability, and functional performance of key phospholipid-based nanocarriers—including liposomes, transferosomes, ethosomes, invasomes, phytosomes, pharmacosomes, and virosomes. We analyze critical structural parameters such as bilayer packing, surface charge, curvature elasticity, and membrane permeability, emphasizing their impact on drug loading efficiency, controlled release, and bioavailability. Advanced multilamellar systems such as vesosomes and spongosomes are also highlighted for their promise in achieving site-specific, sustained drug delivery. Key fabrication methods—including thin-film hydration, ethanol injection, freeze–thaw cycles, and microfluidics—are discussed alongside analytical techniques such as dynamic light scattering (DLS), differential scanning calorimetry (DSC), Fourier-transform infrared spectroscopy (FTIR), and cryo-transmission electron microscopy (cryo-TEM). The review further explores the translational landscape, with a focus on clinically approved liposomal formulations, patent developments, and emerging clinical trials involving stimuli-responsive systems. Persistent challenges such as colloidal stability, tumor penetration, immune system interactions, and scalable manufacturing are critically assessed. Altogether, this review offers a chemistry-focused framework for the rational design and clinical translation of phospholipid nanocarriers in cancer drug delivery.
基于磷脂的靶向肿瘤纳米载体的合理设计与转化研究进展
磷脂衍生的纳米载体代表了一种通用的、化学上可定制的药物输送系统,由于其固有的两亲性,它们可以自组装成双层囊泡。这些系统可以通过非共价相互作用和操纵脂相行为来封装亲水性和疏水性药物。本文综述了磷脂基纳米载体的形成、稳定性和功能性能的分子和超分子原理,这些纳米载体包括脂质体、转移体、质体、侵入体、磷脂质体、药物质体和病毒体。我们分析了关键的结构参数,如双层包装、表面电荷、曲率弹性和膜通透性,强调了它们对药物装载效率、控释和生物利用度的影响。先进的多层系统,如囊体和海绵体,也因其在实现位点特异性、持续给药方面的前景而受到重视。关键的制造方法-包括薄膜水化,乙醇注射,冻融循环和微流体-讨论了分析技术,如动态光散射(DLS),差示扫描量热法(DSC),傅里叶变换红外光谱(FTIR)和低温透射电子显微镜(cro - tem)。这篇综述进一步探讨了转化前景,重点是临床批准的脂质体配方、专利开发和涉及刺激反应系统的新兴临床试验。对胶体稳定性、肿瘤穿透性、免疫系统相互作用和可扩展制造等持续挑战进行了严格评估。总之,这篇综述为合理设计和临床转化用于癌症药物递送的磷脂纳米载体提供了一个以化学为重点的框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


