Formoterol, a clinically approved drug, inhibits ferroptosis by suppressing lipid peroxidation and attenuates APAP-induced acute liver injury
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ferroptosis is an iron-dependent form of regulated cell death characterized by lethal lipid peroxidation and implicated in various human diseases. Despite intensive research, clinically applicable ferroptosis inhibitors remain unavailable. In this study, we identify formoterol, a β2-adrenergic agonist widely used to treat asthma and COPD, as a potent and selective ferroptosis inhibitor through scaffold-based screening of FDA-approved drugs. Formoterol confers robust protection against ferroptotic cell death induced by diverse triggers across multiple human and rodent cell lines, functioning independently of classical pathways such as GPX4, FSP1, or iron chelation. Mechanistic studies reveal that formoterol suppresses ferroptosis by directly scavenging lipid peroxyl radicals, mediated by its unique ortho-amino phenol moiety. In vivo, formoterol significantly alleviates acetaminophen-induced acute liver injury, reducing hepatic lipid peroxidation and preserving tissue integrity. These findings establish formoterol as a clinically approved agent with previously unrecognized anti-ferroptotic activity, offering a compelling example of drug repurposing to accelerate the development of ferroptosis-targeted therapies.
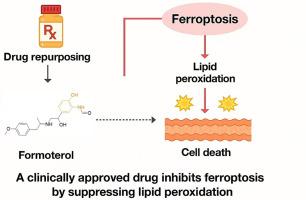
福莫特罗是一种临床批准的药物,通过抑制脂质过氧化来抑制铁下垂,减轻apap引起的急性肝损伤
铁死亡是一种铁依赖性的细胞死亡形式,以致命的脂质过氧化为特征,与各种人类疾病有关。尽管深入的研究,临床上适用的铁下垂抑制剂仍然没有。在这项研究中,我们通过对fda批准的药物进行支架筛选,确定了福莫特罗(一种β2-肾上腺素能激动剂,广泛用于治疗哮喘和慢性阻塞性肺病)是一种有效的选择性铁凋亡抑制剂。福莫特罗对多种人类和啮齿动物细胞系中多种触发因素诱导的铁致细胞死亡具有强大的保护作用,其作用独立于GPX4、FSP1或铁螯合等经典途径。机制研究表明,福莫特罗通过其独特的邻氨基酚部分直接清除脂质过氧化自由基来抑制铁下沉。在体内,福莫特罗显著减轻对乙酰氨基酚引起的急性肝损伤,减少肝脂质过氧化,保持组织完整性。这些发现确立了福莫特罗作为一种临床批准的药物,具有以前未被认识到的抗衰铁活性,为加速衰铁靶向治疗的药物重新定位提供了一个令人信服的例子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


