Apolipoprotein E receptor 2 in endothelium promote glucose tolerance by mediating insulin delivery to skeletal muscle
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
The delivery of circulating insulin to skeletal muscle myocytes is a rate-limiting step in peripheral insulin action, and there is minimal understanding of the underlying mechanisms in endothelial cells. Recognizing that the LDL receptor family member apolipoprotein E receptor 2 (ApoER2, also known as LRP8) mediates apolipoprotein E (ApoE)-induced signaling in endothelial cells, the present project determined if endothelial ApoER2 influences glucose homeostasis in mice.
Methods
Mice were generated deficient in ApoER2 selectively in endothelial cells, and glucose homeostasis was studied. Insulin-stimulated recruitment of the skeletal muscle microvasculature was assessed using contrast-enhanced ultrasound imaging. Endothelial cell insulin uptake and transcytosis were evaluated in culture. The ApoER2 interactome in endothelial cells was interrogated using immunoprecipitation and liquid chromatography/tandem mass spectrometry. ApoER2 structure–function was studied by mutagenesis.
Results
Mice deficient in endothelial cell ApoER2 are glucose intolerant and insulin resistant due to a blunting of skeletal muscle glucose disposal that is related to a decrease in muscle insulin delivery. Endothelial ApoER2 manipulation does not alter direct insulin action on skeletal muscle or insulin-stimulated recruitment of the skeletal muscle microvasculature. Instead, ApoER2 stimulation by apolipoprotein E3 (ApoE3) increases endothelial cell insulin uptake and transcytosis. ApoE3 and ApoER2 stimulation of endothelial insulin transport require the ApoER2 adaptor protein Dab2 and the scaffolding protein IQGAP1, which is known to mediate insulin secretion by pancreatic β cells. IQGAP1 is not required for ApoE3/ApoER2-induced insulin uptake by endothelial cells; alternatively it is necessary for insulin transcytosis. ApoE3 prompts IQGAP1 recruitment to the exocyst complex, and ApoER2 interaction with IQGAP1 is necessary for the recruitment.
Conclusions
In endothelial cells the ApoE3 and ApoER2 tandem co-opts the role of IQGAP1 in pancreatic β cell insulin secretion to enhance endothelial insulin transport. In this manner endothelial ApoER2 promotes glucose disposal in skeletal muscle and supports normal glucose homeostasis.
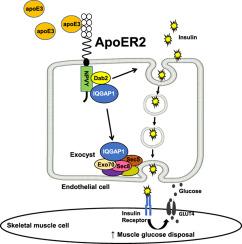
内皮载脂蛋白E受体2通过介导胰岛素传递到骨骼肌促进葡萄糖耐量。
目的:循环胰岛素向骨骼肌肌细胞的传递是外周胰岛素作用的限速步骤,对内皮细胞的潜在机制了解甚少。认识到LDL受体家族成员载脂蛋白E受体2 (ApoER2,也称为LRP8)在内皮细胞中介导载脂蛋白E (ApoE)诱导的信号传导,本项目确定内皮ApoER2是否影响小鼠的葡萄糖稳态。方法:在小鼠内皮细胞中选择性产生ApoER2缺陷,研究葡萄糖稳态。使用超声造影评估胰岛素刺激的骨骼肌微血管募集。内皮细胞胰岛素摄取和胞吞作用在培养中被评估。采用免疫沉淀和液相色谱/串联质谱法研究内皮细胞中的ApoER2相互作用组。通过诱变研究ApoER2的结构和功能。结果:内皮细胞ApoER2缺乏的小鼠出现葡萄糖不耐受和胰岛素抵抗,这是由于骨骼肌葡萄糖处理的钝化,这与肌肉胰岛素输送的减少有关。内皮ApoER2操作不会改变胰岛素对骨骼肌的直接作用或胰岛素刺激骨骼肌微血管的募集。相反,载脂蛋白E3 (ApoE3)刺激ApoER2增加内皮细胞胰岛素摄取和胞吞作用。ApoE3和ApoER2刺激胰岛素内皮转运需要ApoER2的衔接蛋白Dab2和支架蛋白IQGAP1,这两种蛋白介导胰腺β细胞的胰岛素分泌。内皮细胞摄取ApoE3/ apoer2诱导的胰岛素不需要IQGAP1;或者,它是胰岛素胞吞作用所必需的。ApoE3促使IQGAP1向胞囊复合体募集,ApoER2与IQGAP1的相互作用是募集的必要条件。结论:在内皮细胞中,ApoE3和ApoER2串联协同IQGAP1在胰腺β细胞胰岛素分泌中的作用,增强内皮细胞胰岛素转运。通过这种方式,内皮ApoER2促进骨骼肌中的葡萄糖处理并支持正常的葡萄糖稳态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


