Effects of aging and anti-aging dietary restriction on regulators of the [NADPH]/[NADP+] in different neural cell types and brain regions
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Dietary restriction (DR), which slows aging, increases the ratio of reduced glutathione (GSH) to oxidized glutathione disulfide (GSSG) in the brain. DR increases liver cytoplasmic [NADPH]/[NADP+] where much of the NADPH is generated by the folate cycle. This could also occur in astrocytes, the neural cell type with the highest folate cycle flux. Mice on a DR diet showed increased expression of folate cycle enzyme MTHFD1L in several brain regions and likely show increased astrocyte sarcosine catabolism increasing folate cycle cytoplasmic NADPH generation by ALDH1L1. Fasting also increases blood malate/pyruvate that increases tissue [NADPH]/[NADP+]. These events together with decreased NADPH-utilizing lipid synthesis during DR could lead to an increased brain cytoplasmic [NADPH]/[NADP+]. The more reduced NADP(H) pool, combined with the increased expression of brain glutathione disulfide reductase (GSR) and the decreased brain mitochondrial H2O2 generation, decreasing H2O2-induced oxidation of GSH, could lead to the increased brain GSH/GSSG. Aging also decreased the expression of mouse hippocampal NAD+ kinase (NADK) that was restored by DR. Studies that measure the [NADPH]/[NADP+], cysteine/cystine, and GSH/GSSG in different brain regions, subcellular compartments, and neural cell types, especially in astrocytes, during aging and DR are needed to establish effective targets and therapies for aging-related disorders.
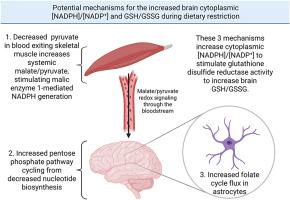
衰老和抗衰老饮食限制对不同神经细胞类型和脑区[NADPH]/[NADP+]调节因子的影响
饮食限制(DR)可以延缓衰老,增加大脑中还原性谷胱甘肽(GSH)与氧化性谷胱甘肽二硫(GSSG)的比例。DR增加肝细胞质[NADPH]/[NADP+],其中大部分NADPH是由叶酸循环产生的。这也可能发生在星形胶质细胞中,这是叶酸循环通量最高的神经细胞类型。DR饮食的小鼠在几个脑区显示叶酸循环酶MTHFD1L的表达增加,并且可能显示星形胶质细胞肌氨酸分解代谢增加,叶酸循环细胞质中ALDH1L1产生的NADPH增加。禁食也会增加血液中的苹果酸/丙酮酸,从而增加组织[NADPH]/[NADP+]。这些事件与DR期间利用NADPH的脂质合成减少一起可能导致脑细胞浆[NADPH]/[NADP+]增加。NADP(H)池减少,脑谷胱甘肽二硫还原酶(GSR)表达增加,脑线粒体H2O2生成减少,H2O2诱导的GSH氧化减少,可导致脑GSH/GSSG增加。衰老还降低了DR恢复的小鼠海马NAD+激酶(NADK)的表达,需要研究衰老和DR过程中不同脑区、亚细胞区室和神经细胞类型(尤其是星形胶质细胞)中[NADPH]/[NADP+]、半胱氨酸/胱氨酸和GSH/GSSG的变化,以建立衰老相关疾病的有效靶点和治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


