A new vapor pressure equation with accurate extrapolation capabilities
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The vapor pressure equation is an effective tool for describing the pressure-temperature relationship along the liquid-vapor coexistence curve. However, most existing equations are merely used to correlate experimental data, but lack reliability when extrapolating beyond the experimental temperature range. Based on the thermodynamic characteristics of vapor pressure, this study summarizes the mathematical constraints of vapor pressure equation, and proposes a new, accurate and reliable vapor pressure equation with four fitting parameters for the correlation and extrapolation of experimental data. The new equation is compared with the Park equation and Wagner equation in terms of correlation and extrapolation performance using experimental data of 62 fluids from the triple point to the critical point. Results show that the new equation matches or slightly outperforms the Park equation and Wagner equation in correlation accuracy, while it has more regular fitting parameters. In terms of extrapolation, the new equation significantly outperforms the Park equation and Wagner equation without parameter constraints, especially towards low-temperature and low-pressure region. And the new equation presents stable extrapolation across different data ranges, achieving a balance between correlation accuracy and extrapolation ability through rigorously validated function forms and reasonably set fitting parameters.
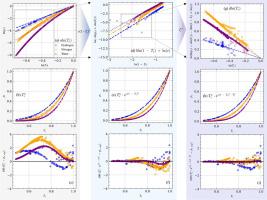
一个具有精确外推能力的新蒸汽压方程
蒸汽压方程是描述液汽共存曲线上压力-温度关系的有效工具。然而,现有的大多数方程仅用于关联实验数据,在外推超出实验温度范围时缺乏可靠性。基于蒸汽压的热力学特性,总结了蒸汽压方程的数学约束,提出了一种新的、准确可靠的、包含4个拟合参数的蒸汽压方程,用于实验数据的相关和外推。利用从三相点到临界点的62种流体的实验数据,将新方程与Park方程和Wagner方程在相关性和外推性能方面进行了比较。结果表明,新方程的相关精度与Park方程和Wagner方程相当或略好,但拟合参数更为规则。外推方面,新方程明显优于无参数约束的Park方程和Wagner方程,特别是在低温低压区域。通过严格验证的函数形式和合理设置的拟合参数,新方程在不同数据范围内具有稳定的外推性,实现了相关精度和外推能力的平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


