Decreasing H3K27me3 alleviates cerebral ischemia/reperfusion injury by modulating FOXP1 expression
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Elevated H3K27me3 levels during cerebral I/R injury exacerbate neuronal damage through oxidative stress, but the underlying mechanism remains to be elucidated. We hypothesized that reduced H3K27me3 confers protection by modulating FOXP1 expression. Employing multifaceted approaches, we demonstrate that H3K27me3 reduction in vivo and in vitro enhances lipid metabolism and rescues oxygen-glucose deprivation (OGD)-induced mitochondrial morphological abnormalities and functional deficits. Furthermore, chromatin immunoprecipitation sequencing analysis revealed that H3K27me3 directly targets FOXP1, a member in the negative regulation of intracellular steroid signal pathway. Further study suggested that genetic knockdown of FOXP1 abolished the protective effects of H3K27me3 reduction against I/R injury. Collectively, our findings establish H3K27me3-dependent FOXP1 repression as a central mechanism driving lipid metabolic dysregulation and mitochondrial dysfunction in cerebral I/R pathogenesis, revealing novel therapeutic targets.
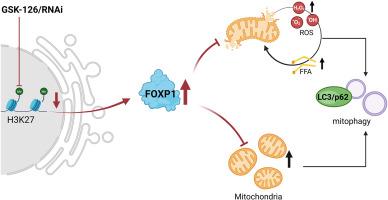
降低H3K27me3通过调节FOXP1表达减轻脑缺血再灌注损伤
脑I/R损伤过程中H3K27me3水平升高通过氧化应激加剧神经元损伤,但其机制尚不清楚。我们假设减少的H3K27me3通过调节FOXP1的表达来提供保护。采用多方面的方法,我们证明了体内和体外H3K27me3的减少增强了脂质代谢,并挽救了氧葡萄糖剥夺(OGD)诱导的线粒体形态异常和功能缺陷。此外,染色质免疫沉淀测序分析显示,H3K27me3直接靶向细胞内类固醇信号通路负调控成员FOXP1。进一步的研究表明,FOXP1基因敲低可消除H3K27me3还原对I/R损伤的保护作用。总之,我们的研究结果确立了h3k27me3依赖性FOXP1抑制是脑I/R发病机制中驱动脂质代谢失调和线粒体功能障碍的中心机制,揭示了新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


