Combined effects of hypoxia and thermal stress on hemocyte response in Pacific oysters (Crassostrea gigas): Insights from transcriptomic and proteomic analyses
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-09-03
DOI:10.1016/j.cbd.2025.101626
引用次数: 0
Abstract
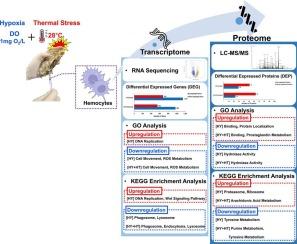
Hypoxia and elevated seawater temperatures are increasingly prevalent stressors in marine ecosystems, significantly impacting the physiology of marine organisms. This study investigates the transcriptomic and proteomic responses of Pacific oyster (Crassostrea gigas) hemocytes to hypoxia alone (water temperature, 23 °C; dissolved oxygen [DO] level, 1 mg O₂/L) and combined hypoxia with high temperature (water temperature, 28 °C; DO level, 1 mg O₂/L) over a 10-day exposure period. Using RNA sequencing and liquid chromatography-mass spectrometry, we identified distinct molecular responses to these stressors. Hypoxia alone upregulated genes associated with DNA replication and protein localization, while suppressing those involved in metabolic activity, reactive oxygen species (ROS) generation, and phagosome function. Under combined hypoxia and high temperature stress, these effects were more pronounced, with enhanced downregulation of hydrolase activity and tyrosine metabolism, indicating synergistic impacts on oyster physiology. Notably, prostaglandin (PG) synthesis was specifically induced under combined stress conditions, suggesting its potential as a biomarker for environmental stress. These findings highlight how hypoxia and thermal stress interact to compromise immune function and energy metabolism in C. gigas, providing critical insights into the physiological vulnerabilities of commercially important bivalves under climate change.
缺氧和热应激对太平洋牡蛎(长牡蛎)血细胞反应的综合影响:转录组学和蛋白质组学分析的见解
缺氧和海水温度升高是海洋生态系统中日益普遍的应激源,对海洋生物的生理产生了重大影响。本研究研究了太平洋牡蛎(长牡蛎)血细胞对缺氧(水温,23°C;溶解氧[DO]水平,1 mg O₂/L)和高温联合缺氧(水温,28°C; DO水平,1 mg O₂/L) 10天暴露期的转录组学和蛋白质组学反应。利用RNA测序和液相色谱-质谱分析,我们确定了对这些应激源的不同分子反应。仅缺氧就上调了与DNA复制和蛋白质定位相关的基因,同时抑制了与代谢活性、活性氧(ROS)产生和吞噬体功能相关的基因。在低氧和高温联合胁迫下,这些影响更为明显,水解酶活性和酪氨酸代谢的下调增强,表明对牡蛎生理有协同作用。值得注意的是,前列腺素(PG)的合成在复合应激条件下被特异性诱导,这表明它有可能作为环境应激的生物标志物。这些发现强调了缺氧和热应激如何相互作用,损害C. gigas的免疫功能和能量代谢,为了解气候变化下商业上重要的双壳类动物的生理脆弱性提供了重要的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


