Combined effects of fulvic acid and phosphate on FeII oxidation and Fe precipitation
引用次数: 0
Abstract
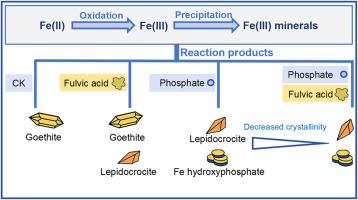
The chemical transformation of ferrous Fe (FeII) under aerobic conditions is a key process governing Fe cycling in soil and is strongly influenced by coexisting constituents such as phosphate and organic matter. Transient redox fluctuations result in the simultaneous presence of FeII and O2. However, the combined effects of phosphate and fulvic acid (FA) on FeII oxidation in the presence of O2 and Fe precipitation remain poorly understood. In this study, we used kinetic experiments and solid-phase characterizations to unravel the underlying mechanisms. Our results demonstrate that FA impeded both FeII oxidation and Fe precipitation. This inhibition might be attributed to FeII complexation with FA and the subsequent adsorption of FA onto Fe mineral surfaces. When phosphate and FA coexisted, phosphate partially decreased the inhibitory FA effects, which is likely due to the formation of Fe hydroxyphosphate and competitive adsorption between phosphate and FA on Fe mineral surfaces. In the absence of phosphate, goethite was the predominant ferric (FeIII) product. However, phosphate addition favored the formation of lepidocrocite and Fe hydroxyphosphate. Increasing phosphate concentrations led to greater phosphate incorporation into Fe minerals. When both phosphate and FA were present, the crystallinity of lepidocrocite and Fe hydroxyphosphate decreased compared to the sole phosphate system. Overall, this study reveals that FA simultaneously inhibited FeII oxidation and Fe precipitation, and that phosphate mitigated these effects via Fe hydroxyphosphate formation and competitive interactions. These findings provide an insight into how coexisting organic and inorganic ligands regulate Fe redox kinetics and Fe precipitation, and underscore the importance of incorporating such interactions into future studies of Fe cycling under fluctuating redox conditions in soil.
黄腐酸和磷酸盐对FeII氧化和铁沉淀的联合影响
铁在好氧条件下的化学转化是控制土壤铁循环的关键过程,并且受到磷酸盐和有机质等共存成分的强烈影响。瞬时氧化还原波动导致FeII和O2同时存在。然而,在O2和Fe沉淀存在的情况下,磷酸盐和富里酸(FA)对FeII氧化的联合影响尚不清楚。在这项研究中,我们使用动力学实验和固相表征来揭示潜在的机制。我们的研究结果表明,FA阻碍了FeII氧化和Fe沉淀。这种抑制作用可能归因于FeII与FA的络合以及随后FA在Fe矿物表面的吸附。当磷酸盐和FA共存时,磷酸盐部分降低了FA的抑制作用,这可能是由于铁羟基磷酸的形成以及磷酸盐和FA在铁矿物表面的竞争性吸附。在没有磷酸盐的情况下,针铁矿是主要的铁(FeIII)产物。然而,磷酸盐的加入有利于鳞片石和铁羟基磷酸的形成。磷酸盐浓度的增加导致更多的磷酸盐掺入到铁矿物中。当磷酸盐和FA同时存在时,蛭石和羟基磷酸铁的结晶度比单一磷酸盐体系降低。总的来说,本研究表明,FA同时抑制FeII氧化和Fe沉淀,而磷酸盐通过铁羟基磷酸盐的形成和竞争相互作用减轻了这些影响。这些发现提供了对共存的有机和无机配体如何调节铁氧化还原动力学和铁沉淀的见解,并强调了将这种相互作用纳入未来土壤中波动氧化还原条件下铁循环研究的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


