Via Proteins and Lipids - Versatility of VAPs at Dynamic Membrane Contact Sites.
Contact (Thousand Oaks (Ventura County, Calif.))
Pub Date : 2025-08-26
eCollection Date: 2025-01-01
DOI:10.1177/25152564251372673
引用次数: 0
Abstract
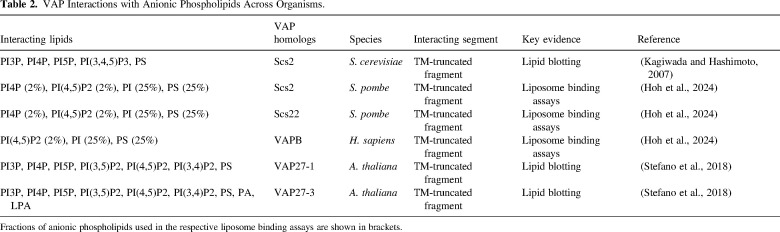
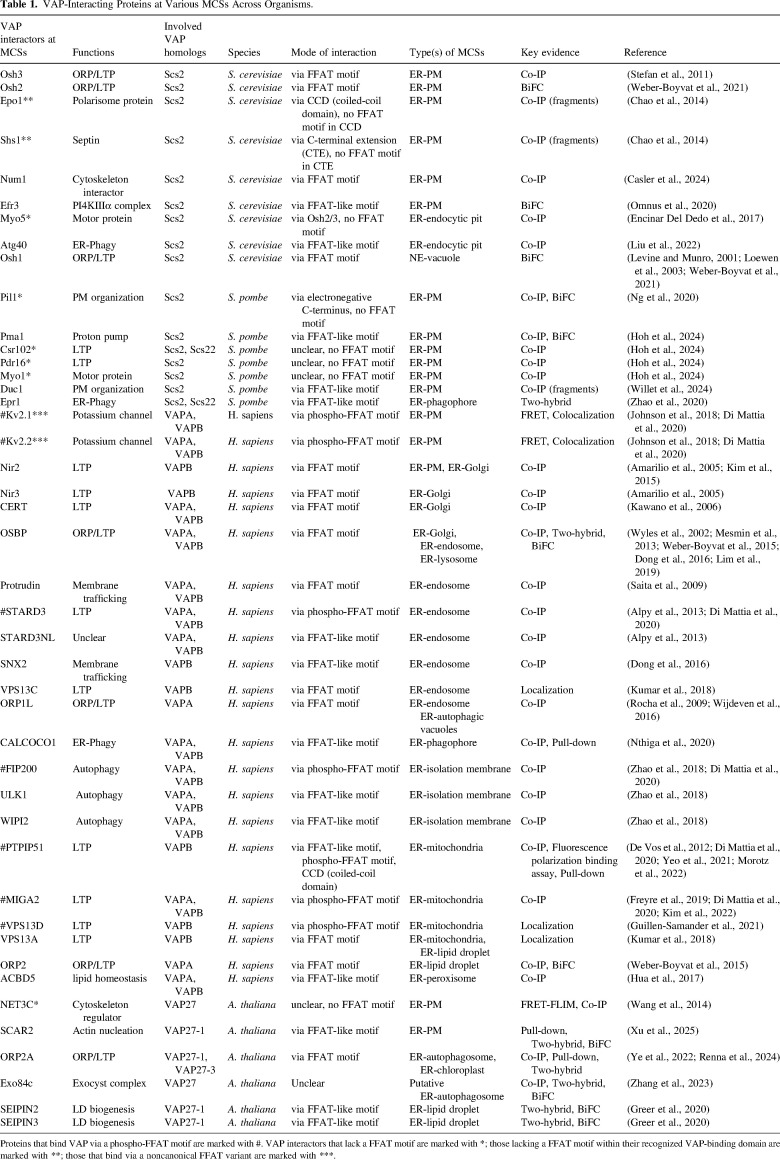
VAMP-associated proteins (VAPs) are highly conserved, endoplasmic reticulum (ER)-resident receptors that tether the ER to various membrane compartments in eukaryotic cells. Each VAP contains a transmembrane helix at its extreme C-terminus and a conserved N-terminal major sperm protein (MSP) domain that mediates various cytosolic interactions via both protein and lipid binding. Here, I question the fundamental difference between protein- and lipid-based associations in VAP-driven membrane contact site (MCS) formation and function - could the lipid affinity of VAPs be an overlooked factor in MCS dynamic regulation?
通过蛋白质和脂质- VAPs在动态膜接触部位的多功能性。
vamp相关蛋白(VAPs)是高度保守的内质网(ER)驻留受体,在真核细胞中将内质网连接到各种膜室。每个VAP在其极端的c端包含一个跨膜螺旋和一个保守的n端主要精子蛋白(MSP)结构域,该结构域通过蛋白质和脂质结合介导各种细胞质相互作用。在这里,我质疑在VAPs驱动的膜接触位点(MCS)形成和功能中基于蛋白质和基于脂质的关联之间的根本区别——VAPs的脂质亲和性是否可能是MCS动态调节中被忽视的因素?
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


