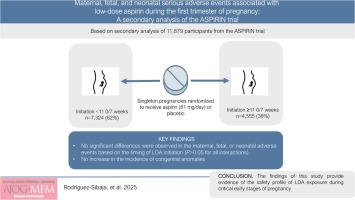
Maternal, fetal, and neonatal serious adverse events associated with low-dose aspirin during the first trimester of pregnancy: A secondary analysis of the Aspirin Supplmentation for Pregnancy Indicated Risk Reduction In Nulliparas (ASPIRIN) trial
IF 3.1
2区 医学
Q1 OBSTETRICS & GYNECOLOGY
American Journal of Obstetrics & Gynecology Mfm
Pub Date : 2025-08-18
DOI:10.1016/j.ajogmf.2025.101768
引用次数: 0
Abstract
Background
Low-dose aspirin (LDA) has been shown to reduce the risk of preterm preeclampsia, particularly when initiated early in pregnancy. However, the safety of starting LDA before 11 0/7 weeks of gestation remains unclear.
Objective
To assess whether initiating LDA before 11 0/7 weeks of gestation is associated with increased maternal, fetal, or neonatal serious adverse events compared to later initiation.
Study Design
This secondary analysis of the ASPIRIN (Aspirin Supplmentation for Pregnancy Indicated Risk Reduction In Nulliparas) trial included 11,879 nulliparous women with singleton pregnancies randomized to receive LDA (81 mg/d) or placebo between 6 0/7–13 6/7 weeks of gestation. Severe adverse events (ie, maternal death, antepartum hemorrhage, postpartum hemorrhage, anemia, preeclampsia or eclampsia, preterm labor, hypertension admission, fever or infection, fetal loss, neonatal death up to 28 days, miscarriage, abortion, or medical termination of pregnancy, and congenital anomalies) were analyzed based on gestational age at LDA initiation (<11 0/7 vs ≥11 0/7 weeks). Furthermore, congenital anomalies were assessed for therapy initiated during the embryonic (ie, <9 0/7 weeks) or fetal period. Interaction tests were performed via logistic regression models on the ASPIRIN trial safety population (ie, participants who received at least one dose of LDA or placebo) and on the subset of participants who had an adherence to the exposure of ≥90%.
Results
Among the 11,879 eligible participants for this secondary analysis, 62% (n=7324) initiated the allocated exposure before 11 0/7 weeks vs 38% (n=4555) that initiated LDA or placebo at a later gestational age. Furthermore, in this population, 84.4% (n=10,030) had an adherence to the intervention of 90% or more. Moreover, the proportion of adherence of ≥90% to LDA or placebo was similar between strata (84.6% [n=6195] for <11 0/7 vs 84.2% [n=3835] ≥11 0/7 weeks, P=.570). No significant differences were observed in the maternal, fetal, or neonatal adverse events described above based on the timing of LDA initiation (P>.05 for all interactions). Likewise, congenital anomalies did not significantly differ between embryonic and fetal exposure periods (interaction P-value = .095). Results remained consistent in participants who had an adherence to the exposure of ≥90%.
Conclusions
LDA (81 mg/d) initiated before 11 0/7 weeks of gestation may not increase the risk of maternal, fetal, or neonatal serious adverse events or congenital anomalies. These findings provide reassuring evidence of the safety of LDA exposure during early pregnancy.
妊娠前三个月与低剂量阿司匹林相关的孕产妇、胎儿和新生儿严重不良事件:阿司匹林试验的二次分析
背景:低剂量阿司匹林(LDA)已被证明可以降低早产子痫前期的风险,特别是在妊娠早期开始服用时。然而,在妊娠11 /7周之前开始LDA的安全性仍不清楚。目的:评估在妊娠11 /7周之前开始LDA与之后开始相比是否与母体、胎儿或新生儿严重不良事件增加相关。研究设计:这项阿司匹林试验的二级分析包括11,879名未生育的单胎妊娠妇女,在妊娠6/7 -13 /7周期间随机接受LDA (81 mg/天)或安慰剂。严重不良事件(即产妇死亡、产前出血、产后出血、贫血、子痫前期或子痫、早产、高血压入院、发热或感染、胎儿丢失、28天以内新生儿死亡、流产、流产或药物终止妊娠、先天性异常)根据LDA起始时的胎龄进行分析(结果:在11,879名符合二级分析条件的参与者中,62% (n= 7324)在11 /7周之前开始分配暴露,38% (n=4,555)在孕晚期开始使用LDA或安慰剂。此外,在该人群中,84.4% (n=10,030)的干预依从性达到90%或更高。此外,LDA或安慰剂依从性≥90%的比例在各阶层之间相似(84.6% [n= 6195],所有相互作用0.05)。同样,先天性异常在胚胎期和胎儿暴露期之间没有显著差异(相互作用p值 = 0.095)。对于依从暴露≥90%的参与者,结果保持一致。结论:妊娠11 /7周前开始的LDA (81 mg/天)可能不会增加母体、胎儿或新生儿严重不良事件或先天性异常的风险。这些发现为妊娠早期暴露于LDA的安全性提供了可靠的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

American Journal of Obstetrics & Gynecology Mfm
Medicine-Medicine (all)
CiteScore
7.40
自引率
3.20%
发文量
254
审稿时长
40 days
期刊介绍:
The American Journal of Obstetrics and Gynecology (AJOG) is a highly esteemed publication with two companion titles. One of these is the American Journal of Obstetrics and Gynecology Maternal-Fetal Medicine (AJOG MFM), which is dedicated to the latest research in the field of maternal-fetal medicine, specifically concerning high-risk pregnancies. The journal encompasses a wide range of topics, including:
Maternal Complications: It addresses significant studies that have the potential to change clinical practice regarding complications faced by pregnant women.
Fetal Complications: The journal covers prenatal diagnosis, ultrasound, and genetic issues related to the fetus, providing insights into the management and care of fetal health.
Prenatal Care: It discusses the best practices in prenatal care to ensure the health and well-being of both the mother and the unborn child.
Intrapartum Care: It provides guidance on the care provided during the childbirth process, which is critical for the safety of both mother and baby.
Postpartum Issues: The journal also tackles issues that arise after childbirth, focusing on the postpartum period and its implications for maternal health. AJOG MFM serves as a reliable forum for peer-reviewed research, with a preference for randomized trials and meta-analyses. The goal is to equip researchers and clinicians with the most current information and evidence-based strategies to effectively manage high-risk pregnancies and to provide the best possible care for mothers and their unborn children.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


