Ceftazidime-Avibactam Pharmacokinetic Comparative In Vivo/In Vitro Study in a Critically Ill Children Under High-Volume Continuous Venovenous Hemodiafiltration.
IF 2.3
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
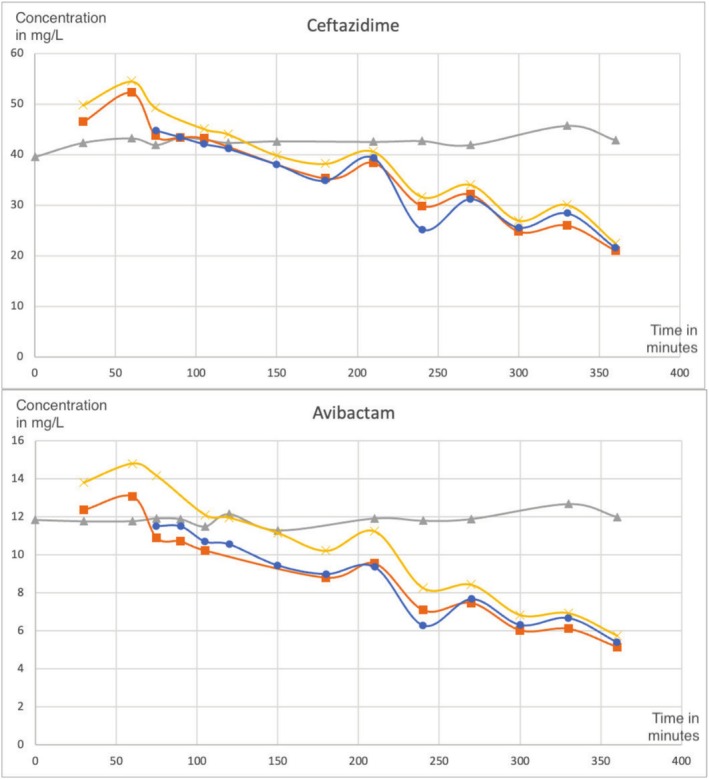
Ceftazidime-avibactam is a novel cephalosporin/beta-lactamase inhibitor combination developed to address increasing antimicrobial resistance. This report presents a comparative study of the pharmacokinetics of ceftazidime and avibactam, utilizing in vitro data derived from two experiments with continuous venovenous hemodiafiltration (CVVHDF) simulation and a comparison with a previously published in vivo case report. The results highlight the importance of therapeutic drug monitoring and the need for higher dosing or continuous infusion of ceftazidime-avibactam in critically ill children under crontinuous renal replacement therapy (CRRT).
头孢他啶-阿维巴坦在重症儿童大容量连续静脉-静脉血液渗滤中的体内/体外比较研究
头孢他啶-阿维巴坦是一种新型头孢菌素/ β -内酰胺酶抑制剂联合开发,以解决日益增加的抗菌素耐药性。本报告对头孢他啶和阿维巴坦的药代动力学进行了比较研究,利用了两个连续静脉静脉血液渗滤(CVVHDF)模拟实验得出的体外数据,并与先前发表的体内病例报告进行了比较。这些结果强调了治疗药物监测的重要性,以及在接受持续肾替代治疗(CRRT)的危重儿童中需要更高剂量或持续输注头孢他啶-阿维巴坦。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacology Research & Perspectives
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
5.30
自引率
3.80%
发文量
120
审稿时长
20 weeks
期刊介绍:
PR&P is jointly published by the American Society for Pharmacology and Experimental Therapeutics (ASPET), the British Pharmacological Society (BPS), and Wiley. PR&P is a bi-monthly open access journal that publishes a range of article types, including: target validation (preclinical papers that show a hypothesis is incorrect or papers on drugs that have failed in early clinical development); drug discovery reviews (strategy, hypotheses, and data resulting in a successful therapeutic drug); frontiers in translational medicine (drug and target validation for an unmet therapeutic need); pharmacological hypotheses (reviews that are oriented to inform a novel hypothesis); and replication studies (work that refutes key findings [failed replication] and work that validates key findings). PR&P publishes papers submitted directly to the journal and those referred from the journals of ASPET and the BPS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


