Slc7a5 promotes T cell anti-tumor immunity through sustaining cytotoxic T lymphocyte effector function
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Tryptophan (Trp) metabolites have emerged as key regulators of host tumor immunity and cancer patient response to immunotherapy. However, the function of and mechanism underlying Trp in tumor-activated CTLs in the tumor microenvironment are incompletely understood. Using a defined co-culture system of tumor-specific CTLs and cognate antigen-expressing tumor cells, we performed a genome-wide metabolomics screening and observed that Trp level is elevated in the tumor cell-activated CTLs. Parallel genome-wide RNA-Sequencing and ATAC-Sequencing analysis determined that tumor-specific CTLs respond to tumor cells by transcriptionally activating Slc7a5 expression. Pharmacological inhibition of Slc7a5 decreased Trp uptake in tumor-activated CTLs and suppressed CTL lytic activity in killing tumor cells in vitro. Mice with Slc7a5 deficiency only in T cells exhibited diminished level of tumor-infiltrating T cells and increased tumor growth and metastasis. scRNA-sequencing analysis revealed that Slc7a5 deficiency resulted in decreased activation of the aryl hydrocarbon receptor (AhR) pathway and repressed FasL expression in tumor-infiltrating T cells. Chromatin immunoprecipitation determined that AhR binds to Faslg promoter in tumor-infiltrating T cells. FasL blockade therapy promotes tumor growth and metastasis in tumor-bearing mice. In human cancer patients, AhR expression correlates with FasL expression in tumor-infiltrating T cells. Furthermore, FasL expression is correlated with patient response to pembrolizumab and survival time. Our finding determines that the Slc7a5-Trp metabolic pathway activates AhR to up-regulate FasL expression in tumor-infiltrating T cells to sustain CTL anti-tumor immunity. Targeting CAR-T cells to up-regulate Slc7a5 to maintain T cell proliferation and function therefore could be a promising direction in cancer immunotherapy.
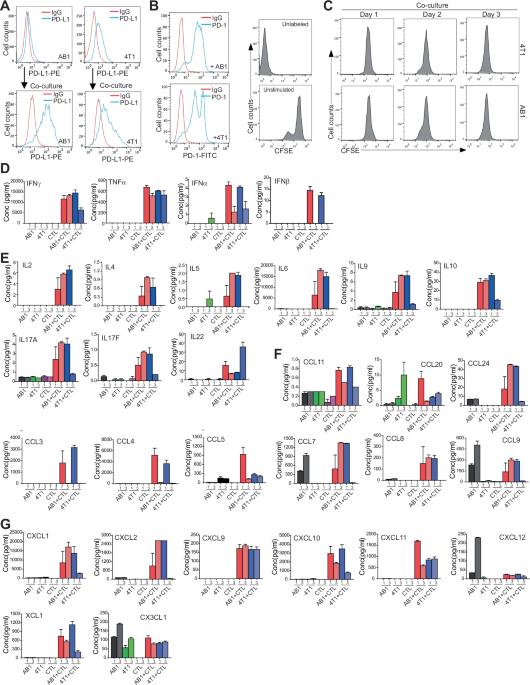
Slc7a5通过维持细胞毒性T淋巴细胞效应功能促进T细胞抗肿瘤免疫。
色氨酸(Trp)代谢物已成为宿主肿瘤免疫和癌症患者对免疫治疗反应的关键调节因子。然而,Trp在肿瘤微环境中肿瘤激活的ctl中的功能和机制尚不完全清楚。使用肿瘤特异性ctl和同源抗原表达肿瘤细胞的共同培养系统,我们进行了全基因组代谢组学筛选,观察到肿瘤细胞激活的ctl中Trp水平升高。平行全基因组rna测序和atac测序分析确定肿瘤特异性ctl通过转录激活Slc7a5表达来响应肿瘤细胞。Slc7a5的药理抑制降低了肿瘤激活的CTL对Trp的摄取,抑制了体外杀伤肿瘤细胞的CTL裂解活性。仅在T细胞中缺乏Slc7a5的小鼠表现出肿瘤浸润T细胞水平降低,肿瘤生长和转移增加。scrna测序分析显示,Slc7a5缺失导致肿瘤浸润性T细胞芳烃受体(AhR)通路激活降低,FasL表达抑制。染色质免疫沉淀测定肿瘤浸润T细胞中AhR与Faslg启动子结合。FasL阻断治疗促进荷瘤小鼠肿瘤生长和转移。在人类癌症患者中,肿瘤浸润T细胞中AhR表达与FasL表达相关。此外,FasL表达与患者对派姆单抗的反应和生存时间相关。我们的发现确定了Slc7a5-Trp代谢途径激活AhR上调肿瘤浸润T细胞中FasL的表达,以维持CTL抗肿瘤免疫。因此,靶向CAR-T细胞上调Slc7a5以维持T细胞的增殖和功能可能是癌症免疫治疗的一个有前途的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


