RCN3 functions as a tumor promoter in colorectal cancer by modulating the GRP78-PI3K-AKT signaling pathway
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Colorectal cancer (CRC) remains a significant global health concern, highlighting the urgent need for novel therapeutic targets and prognostic biomarkers. This study investigates the role of Reticulocalbin-3 (RCN3) in CRC development, focusing on its regulatory influence on calcium ion homeostasis and transport proteins, critical factors in cancer progression. We employed bioinformatics databases, qRT-PCR, Western blotting, and immunohistochemistry to evaluate RCN3 expression levels, revealing that RCN3 is upregulated in CRC tissues and correlates with poorer patient outcomes. Functional assays demonstrated that alterations in RCN3 expression significantly accelerated cell proliferation, migration, and invasion of CRC cells both in vitro and in vivo. Mechanistically, the interaction of RCN3’s EF-hand domains 3 and 4 with glucose-regulated protein 78 (GRP78) promotes its translocation to the cellular membrane, inducing endoplasmic reticulum stress through elevated intracellular calcium levels. Furthermore, RCN3 was found to regulate the PI3K-AKT-mTOR-S6 signaling pathway by enhancing the phosphorylation of key proteins, thereby emphasizing its multifaceted role in CRC pathogenesis. In conclusion, these findings suggest that RCN3 not only contributes to CRC progression but also holds promise as a potential therapeutic target and biomarker for improving patient outcomes in CRC management.
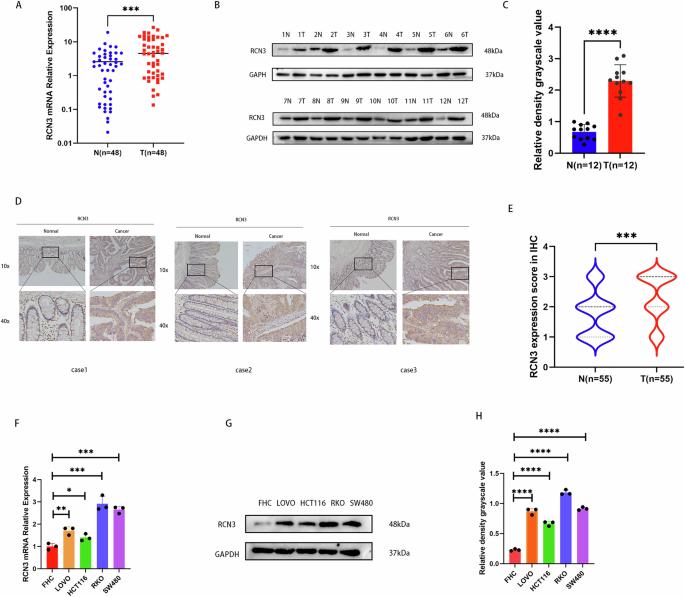
RCN3通过调节GRP78-PI3K-AKT信号通路在结直肠癌中发挥肿瘤启动子的作用。
结直肠癌(CRC)仍然是一个重要的全球健康问题,迫切需要新的治疗靶点和预后生物标志物。本研究探讨了网状定位蛋白-3 (RCN3)在结直肠癌发展中的作用,重点研究了其对钙离子稳态和转运蛋白的调控作用,这是癌症进展的关键因素。我们使用生物信息学数据库、qRT-PCR、Western blotting和免疫组织化学来评估RCN3的表达水平,发现RCN3在结直肠癌组织中表达上调,并与较差的患者预后相关。功能分析表明,RCN3表达的改变在体外和体内都显著加速了CRC细胞的增殖、迁移和侵袭。机制上,RCN3的EF-hand结构域3和4与葡萄糖调节蛋白78 (GRP78)的相互作用促进其向细胞膜的易位,通过提高细胞内钙水平诱导内质网应激。此外,RCN3被发现通过增强关键蛋白的磷酸化来调节PI3K-AKT-mTOR-S6信号通路,从而强调了其在结直肠癌发病中的多方面作用。总之,这些发现表明,RCN3不仅有助于结直肠癌的进展,而且有望成为改善结直肠癌治疗患者预后的潜在治疗靶点和生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


