Suppression of adipocyte ABHD6 favors anti-inflammatory and adipogenic programs to preserve adipose tissue fitness in obesity
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Some individuals exhibit metabolically healthy obesity, characterized by the expansion of white adipose tissue (WAT) without associated complications. The monoacylglycerol (MAG) hydrolase α/β-hydrolase domain-containing 6 (ABHD6) has been implicated in energy metabolism, with its global deletion conferring protection against obesity. However, the immunometabolic roles of adipocyte ABHD6 in WAT remodeling in response to nutri-stress and obesity are not known. Here, we demonstrate that in insulin resistant women, ABHD6 mRNA expression is elevated in visceral fat and positively correlates with obesity and metabolic dysregulation. ABHD6 expression is also elevated in the WATs of diet-induced obese and db/db mice. Although adipocyte-specific ABHD6 knockout (AA-KO) mice become obese under high-fat diet, they show higher plasma adiponectin, reduced circulating insulin and inflammatory markers, improved insulin sensitivity, and lower plasma and liver triglycerides. They also show enhanced insulin action in various tissues, but normal glucose tolerance. In addition, AA-KO mice display healthier and less inflamed expansion of visceral fat, with smaller adipocytes and higher stimulated lipolysis and fatty acid oxidation levels. Similar but less prominent phenotype was found in the subcutaneous and brown fat depots. Thus, adipocyte ABHD6 suppression prevents most of the metabolic and inflammatory complications of obesity, but not obesity per se. Mechanistically, this beneficial process involves a rise in MAG levels in mature adipocytes, and their secretion, resulting in a crosstalk among adipocytes, preadipocytes and macrophages in the adipose microenvironment. Elevated intracellular MAG causes PPARs activation in adipocytes, and MAG secreted from adipocytes curtails the inflammatory polarization of macrophages and promotes preadipocyte differentiation. Hence, adipocyte ABHD6 and MAG hydrolysis contribute to unhealthy WAT remodeling and expansion in obesity, and its suppression represents a candidate strategy to uncouple obesity from many of its immunometabolic complications.
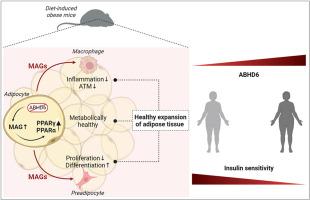
抑制脂肪细胞ABHD6有利于抗炎和脂肪生成程序,以保持肥胖中脂肪组织的健康。
一些个体表现出代谢健康的肥胖,其特征是白色脂肪组织(WAT)的扩张,无相关并发症。单酰基甘油(MAG)水解酶α/β-水解酶结构域6 (ABHD6)与能量代谢有关,其整体缺失可预防肥胖。然而,脂肪细胞ABHD6在WAT重塑中对营养应激和肥胖的免疫代谢作用尚不清楚。在这里,我们证明了在胰岛素抵抗的女性中,ABHD6 mRNA在内脏脂肪中的表达升高,并与肥胖和代谢失调呈正相关。在饮食诱导的肥胖小鼠和db/db小鼠的WATs中,ABHD6的表达也升高。尽管脂肪细胞特异性ABHD6敲除(AA-KO)小鼠在高脂肪饮食下会变得肥胖,但它们表现出更高的血浆脂联素,降低循环胰岛素和炎症标志物,改善胰岛素敏感性,降低血浆和肝脏甘油三酯。它们在各种组织中也表现出增强的胰岛素作用,但葡萄糖耐量正常。此外,AA-KO小鼠表现出更健康和更少的内脏脂肪炎症扩张,脂肪细胞更小,脂肪分解和脂肪酸氧化水平更高。在皮下和棕色脂肪库中发现了类似但不太突出的表型。因此,脂肪细胞ABHD6抑制可以预防肥胖的大多数代谢和炎症并发症,但不能预防肥胖本身。从机制上讲,这一有益的过程涉及成熟脂肪细胞中MAG水平的上升及其分泌,导致脂肪微环境中脂肪细胞、前脂肪细胞和巨噬细胞之间的相互作用。细胞内MAG升高引起脂肪细胞内PPARs活化,脂肪细胞分泌的MAG抑制巨噬细胞的炎症极化,促进前脂肪细胞分化。因此,脂肪细胞ABHD6和MAG水解有助于肥胖中不健康的WAT重塑和扩张,其抑制代表了将肥胖与其许多免疫代谢并发症分离的候选策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


