Modulating metabolism to improve the therapeutic outcomes of CAR cell therapies: From bench to bedside
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Chimeric antigen receptor (CAR) cell therapies have emerged as a groundbreaking approach in cancer treatment, offering new hope for patients with refractory tumors. Despite their success, the therapeutic efficacy of CAR cell therapies is often undermined by metabolic factors within the tumor microenvironment (TME), which impede CAR cell function and lead to treatment resistance. Current literature has not fully explored how these metabolic processes contribute to CAR cell therapy failure, particularly in the context of solid tumors, where the TME presents unique challenges. Addressing this gap is crucial for enhancing the effectiveness of CAR cell therapies across a broader range of cancers. Here, we review the latest research on the metabolic mechanisms that influence CAR cell therapy outcomes, from preclinical studies to clinical applications. We conducted a comprehensive analysis of studies from PubMed and Web of Science, focusing on how various metabolic processes—such as hypoxia, immune cytokine signaling, glycolysis, adenine metabolism, cellular senescence, lactic acid increment, and cholesterol metabolism—affect CAR cell functions, including cytotoxicity, proliferation, stemness, and activation. Additionally, we examine how interactions between CAR cells and other components of the TME, such as tumor cells, stromal cells, and the extracellular matrix, contribute to an immune-suppressive environment that diminishes CAR cell efficacy. We also discuss potential strategies for overcoming these metabolic barriers, including the development of CAR cells with enhanced metabolic regulation, gene expression modulation, and the combination of CAR cell therapy with existing pharmacological treatments. Our findings underscore the critical role of metabolism in shaping the anti-tumor efficacy of CAR cell therapies in both hematologic and solid tumors. By targeting metabolic pathways within the TME, it is possible to enhance CAR cell infiltration, function, and persistence, thereby overcoming resistance and improving therapeutic outcomes. This approach not only addresses a key limitation in current CAR cell therapies but also paves the way for more effective cancer treatments in the future.
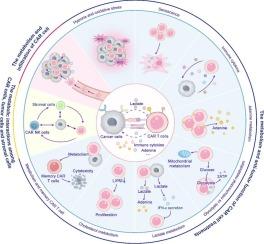
调节代谢以改善CAR细胞疗法的治疗效果:从实验到临床。
嵌合抗原受体(CAR)细胞疗法已经成为一种突破性的癌症治疗方法,为难治性肿瘤患者提供了新的希望。尽管取得了成功,但CAR细胞疗法的治疗效果经常受到肿瘤微环境(TME)内代谢因素的破坏,这些代谢因素阻碍了CAR细胞的功能并导致治疗耐药性。目前的文献并没有充分探讨这些代谢过程是如何导致CAR细胞治疗失败的,特别是在实体肿瘤的背景下,TME提出了独特的挑战。解决这一差距对于提高CAR细胞疗法在更广泛的癌症中的有效性至关重要。在这里,我们回顾了影响CAR细胞治疗结果的代谢机制的最新研究,从临床前研究到临床应用。我们对来自PubMed和Web of Science的研究进行了综合分析,重点关注各种代谢过程(如缺氧、免疫细胞因子信号传导、糖酵解、腺嘌呤代谢、细胞衰老、乳酸增加和胆固醇代谢)如何影响CAR细胞功能,包括细胞毒性、增殖、干细胞和活化。此外,我们还研究了CAR细胞与TME的其他成分(如肿瘤细胞、基质细胞和细胞外基质)之间的相互作用如何导致免疫抑制环境,从而降低CAR细胞的功效。我们还讨论了克服这些代谢障碍的潜在策略,包括开发具有增强代谢调节、基因表达调节的CAR细胞,以及将CAR细胞治疗与现有药物治疗相结合。我们的研究结果强调了代谢在血液和实体肿瘤中形成CAR细胞疗法抗肿瘤疗效的关键作用。通过靶向TME内的代谢途径,有可能增强CAR细胞的浸润、功能和持久性,从而克服耐药性并改善治疗结果。这种方法不仅解决了当前CAR细胞疗法的一个关键限制,而且为未来更有效的癌症治疗铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


