FGF21-loaded M2 macrophage-derived exosomes attenuate sepsis-induced lung injury by regulating M2 macrophage polarization and glycolysis
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Sepsis, a critical lethal critical illness, involves glucose and lipid metabolism disorders. However, the specific mechanisms underlying these alterations require further investigation. Acute lung injury (ALI), one of the most serious complications of sepsis, has a high mortality rate. Fibroblast growth factor 21 (FGF21), a key metabolic regulator, can regulate glucose metabolism. However, the short half-life (t½) of FGF21 limits its clinical application. In this study, we designed FGF21-loaded M2 macrophage-derived exosomes, named FGF21-M2-Exos, for the treatment of sepsis-induced ALI. We demonstrated that FGF21-M2-Exos were able to release FGF21 in a controlled and sustained manner. In addition, FGF21-M2-Exos were present in the lungs of mice at 24 h. Importantly, our results suggest that FGF21-M2-Exos alleviate sepsis-induced lung damage. Moreover, the RNA sequencing results revealed that FGF21-M2-Exos regulate immune function via several inflammatory signalling pathways. Furthermore, we found that FGF21-M2-Exo administration protects against sepsis-induced lung injury by downregulating inflammation-related protein expression, promoting M2 macrophage polarization, inhibiting glycolysis and reducing cellular apoptosis. Overall, the use of FGF21-M2-Exos, which regulate inflammatory and metabolic processes, may be a promising strategy for treating sepsis-induced ALI.
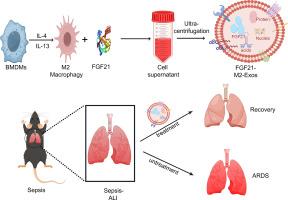
负载fgf21的M2巨噬细胞来源外泌体通过调节M2巨噬细胞极化和糖酵解来减轻败血症诱导的肺损伤。
脓毒症是一种涉及糖脂代谢紊乱的危重致命疾病。然而,这些变化背后的具体机制需要进一步研究。急性肺损伤(ALI)是脓毒症最严重的并发症之一,死亡率很高。成纤维细胞生长因子21 (Fibroblast growth factor 21, FGF21)是一种重要的代谢调节剂,可以调节葡萄糖代谢。然而,FGF21的半衰期短(1 / 2)限制了其临床应用。在本研究中,我们设计了装载fgf21的M2巨噬细胞来源的外泌体,命名为FGF21-M2-Exos,用于治疗败血症诱导的ALI。我们证明了FGF21- m2 - exos能够以可控和持续的方式释放FGF21。此外,FGF21-M2-Exos在24 h时出现在小鼠肺中。重要的是,我们的结果表明FGF21-M2-Exos减轻败血症引起的肺损伤。此外,RNA测序结果显示,FGF21-M2-Exos通过几种炎症信号通路调节免疫功能。此外,我们发现FGF21-M2-Exo通过下调炎症相关蛋白表达、促进M2巨噬细胞极化、抑制糖酵解和减少细胞凋亡来保护败血症诱导的肺损伤。总的来说,使用调节炎症和代谢过程的FGF21-M2-Exos可能是治疗败血症诱导的ALI的一种有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


