NFATc1-targeted siRNA microdroplets prevent radiation-induced osteoporosis by coordinating bone-immune crosstalk
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Radiation-induced bone loss, driven by osteoclast activation, involves the transcription factor nuclear factor of activated T-cells cytoplasmic 1 (NFATc1)-mediated signaling. This study developed NFATc1 siRNA-loaded microdroplets (NFATc1/MDs) to mitigate skeletal damage post-radiotherapy.
Methods
NFATc1/MDs were synthesized and characterized using TEM and confocal microscopy. Biocompatibility was tested in hBMSCs and RAW 264.7 macrophages. Osteoclastogenesis, osteogenesis, and adipogenesis were assessed in vitro, and therapeutic efficacy was evaluated in a rat radiation-induced bone loss model.
Results
NFATc1/MDs exhibited a core-shell structure, high biocompatibility, and efficient cellular uptake. They suppressed osteoclastogenesis without impairing osteogenic/adipogenic differentiation and modulated macrophage activity post-irradiation. In vivo, NFATc1/MDs preserved bone microstructure, reduced osteoclast numbers, and downregulated NFATc1, cathepsin K (CTSK), and tumor necrosis factor-alpha (TNF-α) expression. Conclusions: NFATc1/MDs effectively inhibited osteoclast-mediated bone resorption and inflammation, offering a targeted strategy to prevent radiation-induced bone loss. This biocompatible platform demonstrates potential for clinical translation in radiotherapy-associated skeletal complications.
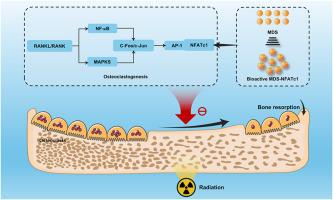
靶向nfatc1的siRNA微滴通过协调骨免疫串扰预防辐射诱导的骨质疏松症。
由破骨细胞激活驱动的辐射致骨丢失,涉及活化t细胞胞质1 (NFATc1)介导的转录因子核因子信号传导。本研究开发了装载NFATc1 sirna的微滴(NFATc1/MDs)来减轻放射治疗后的骨骼损伤。方法:合成NFATc1/MDs,用透射电镜和共聚焦显微镜对其进行表征。在hBMSCs和RAW 264.7巨噬细胞中进行生物相容性测试。在体外评估破骨细胞生成、成骨和脂肪生成,并在大鼠辐射致骨质流失模型中评估治疗效果。结果:NFATc1/MDs具有核壳结构、高生物相容性和高效的细胞摄取。它们抑制破骨细胞生成,但不损害成骨/脂肪分化,并调节照射后巨噬细胞活性。在体内,NFATc1/MDs保留了骨微观结构,减少了破骨细胞数量,下调了NFATc1、组织蛋白酶K (CTSK)和肿瘤坏死因子α (TNF-α)的表达。结论:NFATc1/MDs有效抑制破骨细胞介导的骨吸收和炎症,为预防辐射引起的骨质流失提供了一种有针对性的策略。这种生物相容性平台显示了放射治疗相关骨骼并发症的临床转化潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


