Single-cell, spatial and bulk transcriptome data analysis revealed LINC00467-mediated Sertoli cell ferroptosis is a potential therapeutic target and biomarker for azoospermia
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Human spermatogenesis is an important physiological process related to programmed cell death. However, which type of programmed cell death playing a key role in normal and abnormal human spermatogenesis remains obscure. This study integrated single-cell, bulk RNA and spatial transcriptome data analysis and found that the ferroptosis signal plays a potential role in spermatogenesis and significantly elevate in testicular samples from humans with non-obstructive azoospermia (NOA) due to various factors. In addition, the increased levels of GPX4-dependent ferroptosis in Sertoli cells is a common feature of NOA samples and inhibiting ferroptosis of Sertoli cells in azoospermia patients can improve the functional status of Sertoli cells. Based on weighted correlation network analysis (WGCNA), correlation analysis, differential expression analysis and in vitro experiments, we confirmed that long non-coding RNA (lncRNA) LINC00467 is a key factor in regulating ferroptosis in Sertoli cells. Finally, quantitative real-time polymerase chain reaction (qRT-PCR) experiments showed that LINC00467 can be used to predict sperm retrieval outcomes in NOA patients. In summary, this study reveals and verifies that the level of ferroptosis apparently elevates in Sertoli cells is a novel mechanism for NOA patients, and proposes that ferroptosis-related gene LINC00467 is potential biomarker and therapeutic target for NOA.
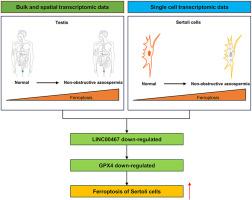
单细胞、空间和大量转录组数据分析显示,linc00467介导的支持细胞铁下垂是无精子症的潜在治疗靶点和生物标志物。
人类精子发生是与细胞程序性死亡相关的重要生理过程。然而,哪种类型的程序性细胞死亡在正常和异常的人类精子发生中起关键作用仍然不清楚。本研究结合单细胞、大体积RNA和空间转录组数据分析发现,在非阻塞性无精子症(NOA)患者的睾丸样本中,铁下沉信号在精子发生中发挥着潜在的作用,并且由于各种因素而显著升高。此外,支持细胞中gpx4依赖性铁下垂水平升高是NOA样本的共同特征,抑制无精子症患者支持细胞的铁下垂可以改善支持细胞的功能状态。基于加权相关网络分析(WGCNA)、相关分析、差异表达分析和体外实验,我们证实长链非编码RNA (lncRNA) LINC00467是调节Sertoli细胞铁下垂的关键因子。最后,定量实时聚合酶链反应(qRT-PCR)实验表明,LINC00467可用于预测NOA患者的精子恢复结果。综上所述,本研究揭示并验证了Sertoli细胞中铁下垂水平明显升高是NOA患者的新机制,并提出了铁下垂相关基因LINC00467是NOA潜在的生物标志物和治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


