LXR-β regulates microglial efferocytosis and neuroinflammation in CPSP via STAT6 activation
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Background
Central post-stroke pain (CPSP) is a chronic neuropathic pain syndrome that develops following cerebrovascular injury and currently lacks effective treatment options. Previous research from our group has found a significant number of apoptotic cells in the thalamus of CPSP rats, and in the nervous system, the failure to promptly clear apoptotic cell debris can activate microglia, triggering a persistent neuroinflammatory response that contributes to the onset and progression of CPSP. Microglia clear apoptotic cells in the central nervous system through efferocytosis, a process that reduces neuroinflammation and promotes the reprogramming of microglia toward the M2 phenotype, which is crucial for immune defense and repair mechanisms in the central nervous system. Recent studies have shown that Liver X Receptor β (LXR-β) can regulate microglial efferocytic function, reduce neuroinflammation after intracerebral hemorrhage, and promote recovery of neurological function. In this study, we explore the potential mechanism by which LXR-β regulates microglial efferocytosis to alleviate CPSP.
Methods
Based on the single-cell sequencing dataset of human brain hemorrhage patients and thalamic tissue samples from rats with central post-stroke pain, a systematic analysis of the dynamic changes in efferocytosis and the associated neuroinflammation was conducted. To verify whether LXR-β regulates CPSP through efferocytosis and its potential mechanism, rats were treated with GW3965 (LXR-β agonist), GSK2033 (LXR-β inhibitor), and AS1517499 (STAT6 inhibitor), either separately or in combination. Assessments included nociceptive behavior, efferocytosis, and the expression of efferocytosis-related molecules, inflammatory factors and microglial polarization markers. In vitro experiments using BV2 cells were also performed to further elucidate the underlying mechanisms.
Results
Human brain hemorrhage sequencing and the CPSP rat thalamic hemorrhage model results indicated that insufficient clearance of apoptotic cells and abnormal activation of microglia were key factors contributing to abnormal neuroinflammation following a stroke. The down-regulation of LXR-β is associated with mechanical allodynia after CPSP. Activation of LXR-β enhanced efferocytosis, and upregulated efferocytosis-related molecules (MerTK, Axl, and CD36). These effects contributed to reduced neuroinflammation, promoted microglial polarization toward the M2 phenotype, and alleviated CPSP. Biological analyses and experimental results indicated that LXR-β regulated these effects through the activation of p-STAT6. In vitro studies also confirmed that the LXR-β/p-STAT6 signaling pathway is closely associated with efferocytosis and inflammation regulation in BV2 cells.
Conclusions
LXR-β promotes microglial efferocytosis and the expression of efferocytosis-related molecules (Mertk, Axl, and CD36) by activating p-STAT6, thereby reducing neuroinflammation, reprogramming microglia toward the M2 phenotype, and alleviating CPSP. Targeting LXR-β or its downstream signaling pathways may offer a promising therapeutic strategy for central neuropathic pain.
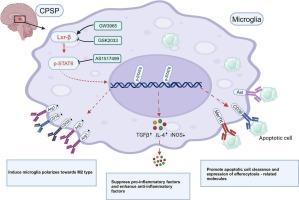
LXR-β通过STAT6激活调节CPSP的小胶质细胞efferocyte和神经炎症。
背景:中枢性卒中后疼痛(CPSP)是脑血管损伤后发生的一种慢性神经性疼痛综合征,目前缺乏有效的治疗方案。本课组前期研究发现,CPSP大鼠的丘脑中存在大量凋亡细胞,而在神经系统中,未能及时清除凋亡细胞碎片可激活小胶质细胞,引发持续的神经炎症反应,促进CPSP的发生和发展。小胶质细胞通过efferocytosis清除中枢神经系统中的凋亡细胞,这一过程可以减少神经炎症并促进小胶质细胞向M2表型重编程,这对于中枢神经系统的免疫防御和修复机制至关重要。近期研究表明,肝脏 X 受体β (LXR-β)可调节小胶质细胞efferocytic功能,减轻脑出血后神经炎症,促进神经功能恢复。在本研究中,我们探讨了LXR-β通过调节小胶质细胞efferocytosis来缓解CPSP的可能机制。方法:基于脑出血患者单细胞测序数据集和中枢性脑卒中后疼痛大鼠丘脑组织样本,系统分析脑卒中后脑出血患者脑内efferocytosis及相关神经炎症的动态变化。为了验证LXR-β是否通过efferocytosis调节CPSP及其潜在机制,我们分别用GW3965 (LXR-β激动剂)、GSK2033 (LXR-β抑制剂)和AS1517499 (STAT6抑制剂)单独或联合治疗大鼠。评估包括伤害性行为、efferocytosis、efferocytosis相关分子、炎症因子和小胶质极化标记物的表达。利用BV2细胞进行的体外实验也进一步阐明了潜在的机制。结果:人脑出血测序和CPSP大鼠丘脑出血模型结果表明,凋亡细胞清除不足和小胶质细胞异常活化是导致脑卒中后神经炎症异常的关键因素。LXR-β的下调与CPSP后的机械异常性疼痛有关。LXR-β的激活增强了efferocytosis,上调了efferocytosis相关分子(MerTK, Axl和CD36)。这些作用有助于减少神经炎症,促进小胶质细胞向M2表型极化,减轻CPSP。生物学分析和实验结果表明,LXR-β通过激活p-STAT6调节这些作用。体外研究也证实LXR-β/p-STAT6信号通路与BV2细胞的efferocytosis和炎症调节密切相关。结论:LXR-β通过激活p-STAT6促进小胶质细胞efferocysis和efferocysis相关分子(Mertk, Axl和CD36)的表达,从而减少神经炎症,使小胶质细胞向M2表型重编程,缓解CPSP。靶向LXR-β或其下游信号通路可能为中枢神经性疼痛提供一种有希望的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


