Unexplored gene PHKA1 interplays between glucose metabolism and breast cancer
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-27
DOI:10.1016/j.bbamcr.2025.120052
引用次数: 0
Abstract
Cancer cells often undergo metabolic reprogramming, typically increasing their uptake and utilization of energy sources like glucose, fatty acids, lactate, glutamine, and pyruvate, while maintaining redox balance. Rather than relying on oxidative phosphorylation, cancer cells preferentially engage glycolysis to convert pyruvate into lactate. This metabolic reprogramming correlates with altered glucose metabolism and dysregulated insulin signalling. Diabetes is associated with increased risk of certain cancer types. Cancer database analysis of genes involved in glucose metabolism, insulin signalling and diabetes, identified an unexplored differentially expressed PHKA1 gene associated with poor patient survivability in breast cancer. Expression of the PHKA1 gene was found to be upregulated under an environment of high glucose and insulin in cancer cells. Silencing PHKA1 via siRNA led to marked decrease in proliferative, invasion, migratory, and stem-like properties of MDA-MB-231 and MCF-7 breast cancer cells. Experimental findings demonstrated reduced expression of mesenchymal markers (e.g., Vimentin, Zeb-1/2), cell cycle markers (e.g., CDK-2/4), and proliferative markers (e.g., Bcl-2 and Bcl-xl), while expression of epithelial markers (e.g., E-cadherin and Keratin-19) were enhanced in PHKA1 knockdown cells when compared to control. Performance of glycolysis stress and mito stress assay further demonstrated that siPHKA1 cells had diminished glycolytic activity alongside suppressed mitochondrial function. These findings highlight intricate relationship between metabolic dysregulation observed in diabetes, contributing to the progression of cancer. Collectively, these observations highlight PHKA1 as an oncogenic candidate with potential role in breast cancer. Comprehensive understanding of such metabolic alterations is critical to designing targeted therapeutic strategies aimed at mitigating breast cancer progression.
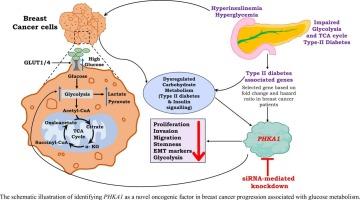
未被发现的基因PHKA1在葡萄糖代谢和乳腺癌之间相互作用。
癌细胞经常经历代谢重编程,通常会增加对葡萄糖、脂肪酸、乳酸、谷氨酰胺和丙酮酸等能量来源的摄取和利用,同时保持氧化还原平衡。癌细胞不依赖于氧化磷酸化,而是优先进行糖酵解,将丙酮酸转化为乳酸。这种代谢重编程与葡萄糖代谢改变和胰岛素信号失调有关。糖尿病会增加患某些癌症的风险。通过对葡萄糖代谢、胰岛素信号传导和糖尿病相关基因的癌症数据库分析,发现了一种未被发现的差异表达的PHKA1基因,该基因与乳腺癌患者生存能力差有关。PHKA1基因在高葡萄糖和胰岛素环境下被发现在癌细胞中表达上调。通过siRNA沉默PHKA1导致MDA-MB-231和MCF-7乳腺癌细胞的增殖、侵袭、迁移和干细胞样特性显著降低。实验结果显示,与对照组相比,PHKA1敲除细胞中间充质标记物(如Vimentin、Zeb-1/2)、细胞周期标记物(如CDK-2/4)和增殖标记物(如Bcl-2和Bcl-xl)的表达降低,而上皮标记物(如E-cadherin和Keratin-19)的表达增强。糖酵解应激和线粒体应激实验进一步表明,siPHKA1细胞糖酵解活性降低,线粒体功能受到抑制。这些发现强调了在糖尿病中观察到的代谢失调与癌症进展之间的复杂关系。总的来说,这些观察结果突出了PHKA1在乳腺癌中具有潜在的致癌作用。全面了解这种代谢改变对于设计旨在缓解乳腺癌进展的靶向治疗策略至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


