The Proteostasis Network in Proteinopathies
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
Proteinopathies are neurodegenerative disorders that are characterized by accumulation of misfolded toxic protein aggregates that lead to synaptic and neuronal dysfunction. Although genetically, clinically, and pathologically distinct, a common feature of these diseases is disruption of protein homeostasis (proteostasis), which causes accumulation of misfolded proteins. The machinery mediating proteostasis exquisitely balances and interlaces protein synthesis, protein folding and trafficking, and protein degradation processes within the proteostasis network to maintain homeostasis. The proteostasis network governs a functional and dynamic proteome by modulating the timing, location, and stoichiometry of protein expression, surveillance, and maintenance of protein folding and removal of misfolded or excess proteins. Although a functional proteome is essential for the health of all cell types, this is especially true for neurons, which are prone to enhanced cellular stress. Aging is the most important risk factor for proteostasis decline and the development of proteinopathies. However, germline and somatic mutations can also functionally impair components of the proteostasis network. Post-mitotic cells, particularly neurons, are rendered further susceptible to proteostasis dysfunction because of their extended lifespan. This review discusses the interconnections between the functional components mediating proteostasis in neuronal cells and how aberrations in proteostasis contribute to neuronal dysfunction and disease.
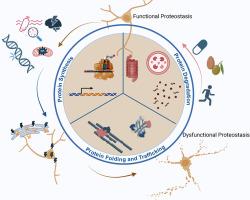
蛋白质病变中的蛋白质抑制网络:机制和相互联系。
蛋白质病是神经退行性疾病,其特征是错误折叠的有毒蛋白质聚集体的积累,导致突触和神经元功能障碍。尽管这些疾病在遗传、临床和病理上是不同的,但它们的一个共同特征是蛋白质稳态(proteostasis)被破坏,导致错误折叠蛋白质的积累。调节蛋白质平衡的机制巧妙地平衡和交错蛋白质合成,蛋白质折叠和运输,以及蛋白质降解过程在蛋白质平衡网络中维持稳态。蛋白质静止网络通过调节蛋白质表达的时间、位置和化学计量,监视和维持蛋白质折叠以及去除错误折叠或过量蛋白质来控制功能和动态蛋白质组。尽管功能性蛋白质组对所有细胞类型的健康都是必不可少的,但对于容易受到细胞压力增强的神经元来说尤其如此。衰老是蛋白质抑制能力下降和蛋白质病变发生的最重要的危险因素。然而,种系和体细胞突变也可以在功能上损害蛋白质静止网络的组成部分。有丝分裂后的细胞,特别是神经元,由于它们的寿命延长,更容易受到蛋白质平衡功能障碍的影响。本文就神经元细胞中介导蛋白质平衡的功能成分之间的相互联系以及蛋白质平衡异常如何导致神经元功能障碍和疾病进行了综述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


