Cryo-EM exposes diverse polymorphism in IAPP mutants to guide the rational design of peptide-based therapeutics
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
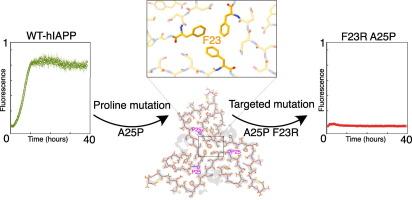
In the pursuit of potential therapeutic agents for type 2 diabetes, non-amyloidogenic forms of the human Islet Amyloid Polypeptide (hIAPP) containing site-specific mutations are of significant interest. In the present study, we dissect the three proline mutations present in the core region of the non-amyloidogenic rat IAPP into single-point mutations at A25P, S28P, and S29P sites. We apply high-resolution cryo-electron microscopy and solve the structures of 6 polymorphs formed by these mutants, revealing the peptide’s self-assembly patterns and identifying critical interactions that reinforce these structures in the presence of the -sheet breaker. A unique trimeric aggregate with C3 symmetry was identified in the A25P mutant, which we resolved with a 3.05 Å resolution, while asymmetric trimeric assemblies were observed in the other mutants. Guided by the high-resolution structural models of A25P and S28P fibrils obtained in our study, we successfully designed novel non-amyloidogenic mutants of IAPP with potential therapeutic value. Our findings demonstrate the immense potential of structure-based approaches in developing effective therapeutics against amyloid diseases.
Cryo-EM揭示了IAPP突变体的多种多态性,以指导基于肽的治疗方法的合理设计。
在寻找2型糖尿病的潜在治疗药物的过程中,含有位点特异性突变的人类胰岛淀粉样多肽(hIAPP)的非淀粉样变性形式引起了人们的极大兴趣。在本研究中,我们将存在于非淀粉样变性大鼠IAPP核心区域的三个脯氨酸突变分解为A25P、S28P和S29P位点的单点突变。我们利用高分辨率冷冻电镜分析了这些突变体形成的6个多态性的结构,揭示了肽的自组装模式,并确定了在β-破片剂存在时加强这些结构的关键相互作用。在A25P突变体中发现了具有C3对称性的独特三聚体聚集体,我们以3.05 Å分辨率对其进行了解析,而在其他突变体中观察到不对称的三聚体聚集体。在本研究获得的A25P和S28P原纤维高分辨率结构模型的指导下,我们成功设计了具有潜在治疗价值的新型IAPP非淀粉样变性突变体。我们的发现证明了基于结构的方法在开发有效治疗淀粉样蛋白疾病方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


