Design, synthesis and evaluation of clickable photoaffinity probes for nuclear lamins
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Nuclear lamins are type V intermediate filament proteins to support the mechanical stability of mammalian cell nucleus. They also participate in various signaling activities in the cells. We recently discovered substituted pyrroloquinazoline LBL1 as the first small molecule to directly target nuclear lamins despite their poor druggability. Based on LBL1, a clickable photoaffinity probe LBL1-PCF was designed to identify lamin-interactors in native cells. In this work, we designed and synthesized a series of clickable photoaffinity probes to evaluate the structure-activity relationships for lamin labeling. Appending an azidopropyl group to the pyrroloquinazoline core at N-7 was well-tolerated without affecting the labeling EC50. Substitution at N-1 significantly reduced its efficiency for lamin labeling. On the other hand, shifting the azidopropyl group to the benzamide at N-3 of pyrroloquinazoline core abolished its capability for lamin labeling. Our results demonstrate that strategic placement of the clickable group at the pyrroloquinazoline core is of paramount importance for target identification studies.
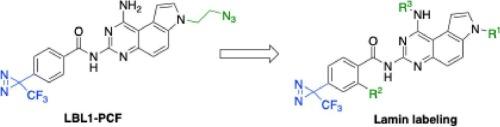
核层蛋白可点击光亲和探针的设计、合成和评价。
核层蛋白是支持哺乳动物细胞核机械稳定性的V型中间丝蛋白。它们还参与细胞内的各种信号活动。我们最近发现取代吡咯喹啉LBL1是第一个直接靶向核层蛋白的小分子,尽管它们的药物性很差。基于LBL1,设计了一种可点击的光亲和探针LBL1- pcf,用于鉴定天然细胞中的层粘连蛋白相互作用物。在这项工作中,我们设计并合成了一系列可点击的光亲和探针,以评估层粘连蛋白标记的结构-活性关系。在吡咯喹啉核心N-7处附加叠氮丙基耐受性良好,不影响标记EC50。N-1的取代显著降低了其对层粘连蛋白的标记效率。另一方面,将叠氮丙基转移到吡咯喹啉核心N-3位的苯酰胺,使其失去了层粘连蛋白的标记能力。我们的研究结果表明,在吡咯喹啉核心的可点击基团的战略位置对目标识别研究至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


