SGK1 inhibits oxidative injury and extracellular matrix degradation by activating the GSK-3β (Ser9)/Fyn/NRF2 pathway in pelvic organ prolapse
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
Sustained oxidative stress (OS) promotes the development of pelvic organ prolapse (POP); however, the pathogenesis of POP under OS conditions remains unclear. This study aimed to investigate the role of serum and glucocorticoid-induced protein kinase 1 (SGK1) in the progression of POP in OS and elucidate its potential molecular mechanisms.
Materials and methods
The protein levels of SGK1 in fibroblasts and other cells within the uterosacral ligament tissues (ULTs) from patients with POP in OS were measured by immunofluorescence (IF). Human uterosacral ligament fibroblasts (hUSLFs) were treated with hydrogen peroxide (H2O2) to establish an in vitro model of oxidative damage. SGK1 was overexpressed and knocked down using a lentivirus to investigate oxidative damage and extracellular matrix (ECM) degradation under H2O2 stimulation. The interaction between SGK1 and GSK-3β was explored using co-immunoprecipitation assays, molecular docking models, and IF.
Key findings
SGK1 was upregulated in fibroblasts within the ULTs from patients with POP under OS conditions and in H2O2-induced hUSLFs. SGK1 overexpression in H2O2-treated hUSLFs inhibited H2O2-triggered apoptosis, reactive oxygen species generation, and collagen loss, whereas SGK1 depletion promoted these processes. Mechanistically, SGK1 suppressed OS-induced oxidative damage, and ECM degradation in hUSLFs by binding with GSK-3β to activate the GSK-3β (Ser9)/Fyn/NRF2 pathway.
Significance
Our results revealed that SGK1 could potentially slow down the progression of POP under OS by interacting with GSK-3β to promote the GSK-3β (Ser9)/Fyn/NRF2 pathway, which provides a potential therapeutic approach for treating POP.
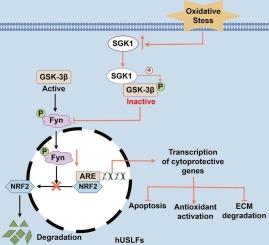
SGK1通过激活盆腔器官脱出中的GSK-3β (Ser9)/Fyn/NRF2通路抑制氧化损伤和细胞外基质降解
目的持续氧化应激(OS)促进盆腔器官脱垂(POP)的发生;然而,OS条件下POP的发病机制尚不清楚。本研究旨在探讨血清和糖皮质激素诱导的蛋白激酶1 (SGK1)在OS中POP进展中的作用,并阐明其潜在的分子机制。材料与方法采用免疫荧光法(IF)检测OS POP患者子宫骶韧带组织(ULTs)成纤维细胞及其他细胞中SGK1蛋白水平。采用过氧化氢(H2O2)处理人子宫骶韧带成纤维细胞(hUSLFs),建立体外氧化损伤模型。在H2O2刺激下,SGK1过表达并被慢病毒敲除,以研究氧化损伤和细胞外基质(ECM)降解。利用共免疫沉淀法、分子对接模型和IF研究了SGK1和GSK-3β之间的相互作用。主要发现ssgk1在OS条件下来自POP患者的ult中的成纤维细胞和h2o2诱导的hUSLFs中上调。在h2o2处理的hUSLFs中,SGK1过表达抑制h2o2引发的细胞凋亡、活性氧生成和胶原流失,而SGK1缺失促进了这些过程。在机制上,SGK1通过与GSK-3β结合激活GSK-3β (Ser9)/Fyn/NRF2通路,抑制os诱导的氧化损伤和hUSLFs中的ECM降解。我们的研究结果表明,SGK1可能通过与GSK-3β相互作用,促进GSK-3β (Ser9)/Fyn/NRF2通路,从而潜在地减缓OS下POP的进展,这为治疗POP提供了潜在的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


