A clinical study on the efficacy of concurrent chemoradiotherapy combined with targeted therapy and hyperthermia in patients with locally advanced cervical cancer
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Objective
To explore novel therapeutic approaches for locally advanced cervical cancer (LACC), we evaluated the efficacy and safety of hyperthermia (HT) and/or targeted therapy combined with cisplatin-based concurrent chemoradiotherapy (CCRT).
Methods
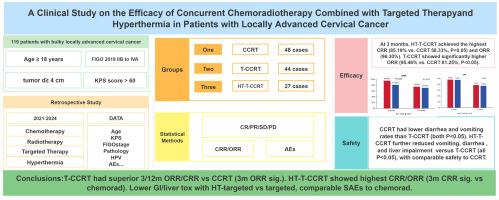
This retrospective study analyzed 119 LACC patients (tumor diameter ≥4 cm) treated at our institution (Jan 2021–Oct 2024), stratified into: CCRT (n = 48), targeted therapy + CCRT (T-CCRT, n = 44), and HT + targeted therapy + CCRT (HT-T-CCRT, n = 27). Complete/objective response rates (CRR/ORR) at 3/12 months were assessed. Statistical analyses were performed using standard software to compare efficacies and evaluate adverse events (AEs).
Results
1. Efficacy: At 3 months, HT-T-CCRT achieved the highest CRR (85.19 % vs. CCRT 58.33 %, P < 0.05) and ORR (96.30 %). T-CCRT showed significantly higher ORR (95.46 % vs. CCRT 81.25 %, P < 0.05). At 12 months, no significant intergroup differences existed in CRR (58.33 %/61.36 %/77.78 %) or ORR (70.83 %/75.00 %/77.78 %). 2. Safety: CCRT had lower diarrhea (52.08 % vs. 81.82 %) and vomiting (50.00 % vs. 86.36 %) rates than T-CCRT (both P < 0.05). HT-T-CCRT further reduced vomiting (55.56 % vs. 86.36 %), diarrhea (55.56 % vs. 81.82 %), and liver impairment (22.22 % vs. 47.73 %) versus T-CCRT (all P < 0.05), with comparable safety to CCRT.
Conclusions
Efficacy: The targeted combination group had higher ORR and CRR at 3/12 months than chemoradiation, with a significant 3-month ORR difference. The thermotherapy-targeted group showed the highest CRR/ORR, with significantly better 3-month CRR vs chemoradiation but not vs targeted alone. Safety: Diarrhea/vomiting were lower with chemoradiation than targeted; thermotherapy-targeted had lower diarrhea/vomiting/liver injury than targeted alone, with comparable overall AEs to chemoradiation.
同步放化疗联合靶向治疗和热疗治疗局部晚期宫颈癌的临床研究
目的探讨局部晚期宫颈癌(LACC)的新治疗方法,评价热疗(HT)和/或靶向治疗联合以顺铂为基础的同步放化疗(CCRT)的疗效和安全性。方法回顾性分析我院(2021年1月- 2024年10月)治疗的119例肿瘤直径≥4cm的LACC患者,分为CCRT (n = 48)、靶向治疗+ CCRT (T-CCRT, n = 44)和HT +靶向治疗+ CCRT (HT-T-CCRT, n = 27)。评估3/12个月的完全/客观缓解率(CRR/ORR)。采用标准软件进行疗效比较和不良事件(ae)评价的统计分析。疗效:3个月时,HT-T-CCRT达到最高的CRR (85.19% vs. CCRT 58.33%, P < 0.05)和ORR(96.30%)。T-CCRT的ORR为95.46%,CCRT为81.25%,P < 0.05)。12个月时,CRR(58.33% / 61.36% / 77.78%)和ORR(70.83% / 75.00% / 77.78%)组间无显著差异。2. 安全性:CCRT的腹泻率(52.08%比81.82%)和呕吐率(50.00%比86.36%)低于T-CCRT (P < 0.05)。与T-CCRT相比,HT-T-CCRT进一步减少了呕吐(55.56%对86.36%)、腹泻(55.56%对81.82%)和肝损害(22.22%对47.73%)(均P <; 0.05),安全性与CCRT相当。结论疗效:靶向联合组3/12月的ORR和CRR均高于放化疗组,3个月ORR差异有统计学意义。热疗靶向组显示出最高的CRR/ORR,与放化疗相比,3个月的CRR明显更好,但与单独靶向组相比则没有。安全性:放化疗组腹泻/呕吐低于靶向放化疗组;与单独靶向治疗相比,靶向热疗法的腹泻/呕吐/肝损伤发生率更低,总体ae与放化疗相当。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


