Design, synthesis, and biological activity of novel para-substituted phenylamino and phenyl derivatives of northebaine as selective delta opioid receptor agonists
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
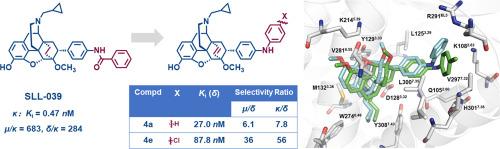
The delta opioid receptor (DOR) is a promising target for developing analgesics with fewer side effects compared to mu opioid receptor (MOR) agonists. However, non-peptidyl DOR-selective agonists remain limited. Using the “message-address” concept in opioid ligand design, we designed and synthesized a series of para-substituted N-cyclopropylmethyl-7α-phenyl-6,14-endoetheno-tetrahydronorthebaines to explore their binding affinity and selectivity for DOR over MOR and kappa opioid receptor (KOR). Key findings revealed that para-substituted phenylamino derivatives exhibited high DOR affinity and subtype selectivity. Functional assays confirmed their agonistic activity at DOR, with compounds 4a and 4e showing IC50 values of 580.9 nM and 4807 nM, respectively. Molecular modeling studies revealed that DOR selectivity might be mediated by specific interactions with residue L3007.35 in the TM7 domain, where structural rearrangement of the address component facilitates its transition from KOR- to DOR-selective binding modes. These findings highlight the critical role of “address” component optimization in achieving receptor subtype specificity, providing a structure-based strategy for developing new opioid therapeutics with tailored pharmacological profiles.
新型对取代苯胺及其苯基衍生物选择性阿片受体激动剂的设计、合成和生物活性研究
与mu阿片受体(MOR)激动剂相比,delta阿片受体(DOR)是开发副作用更小的镇痛药的有希望的靶点。然而,非肽基dor选择性激动剂仍然有限。利用阿片配体设计中的“信息-地址”概念,我们设计并合成了一系列对取代的n -环丙基甲基-7α-苯基-6,14-烯乙基-四氢正啡碱,以探索它们对DOR对MOR和kappa阿片受体(KOR)的结合亲和力和选择性。主要研究结果表明,对取代苯胺衍生物具有高DOR亲和力和亚型选择性。功能分析证实了它们对DOR的激动作用,化合物4a和4e的IC50值分别为580.9 nM和4807 nM。分子模拟研究表明,DOR选择性可能是通过与TM7结构域中L3007.35残基的特异性相互作用介导的,其中地址成分的结构重排促进了其从KOR-选择性结合模式转变为DOR-选择性结合模式。这些发现强调了“地址”成分优化在实现受体亚型特异性中的关键作用,为开发具有量身定制药理学特征的新型阿片类药物提供了基于结构的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


