Aloperine ameliorates radiation enteritis by driving the TAS2R138-PLCβ/GAPDH axis-mediated glucose metabolic reprogramming to inhibit ferroptosis in intestinal epithelial cells
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Radiation enteritis (RE) is a common side effect of radiotherapy, with no specific therapeutic agents available. Sophora alopecuroides L. (KDZ) has been used in China for many years to treat gastrointestinal disorders, and matrine, oxymatrine, and aloperine (ALO) are its main alkaloid components. We found that ALO significantly attenuated the damage of ionizing radiation (IR) to the viability of intestinal epithelial cells (IECs), outperforming matrine and oxymatrine. The IR-induced Fe2+ accumulation, lipid peroxidation and down-regulated glutathione peroxidase 4 and solute carrier family 7 member 11 levels were restored, and ferroptosis but not apoptosis or necrosis was further indicated. Untargeted metabolomic analysis showed that ALO shifted the glucose flux from glycolysis to the hexosamine biosynthetic pathway to elevate the UDP-GlcNAc level. Notably, ALO could directly bind with taste 2 receptor member 138 (TAS2R138) as one of its potential targets, and promoted the activation of downstream signaling molecules phospholipase C β (PLCβ) and α-gustducin. Furthermore, ALO enhanced the association of PLCβ and GAPDH at MET56 and LYS271, and inhibiting the latter’s acetylation and activity via activating TAS2R138. In addition, after triggering the metabolic reprogramming, ALO promoted the O/N-glycosylation to prevent the lysosomal degradation of ferritin heavy chain 1 and transferrin receptor, modulating iron storage and transport, thus alleviating ferroptosis. In vivo, the above-mentioned effect and mechanisms of ALO were demonstrated using the model of RE in mice. Collectively, ALO activated TAS2R138 to promote the interaction of PLCβ and GAPDH to induce metabolic reprogramming and alleviate IR-induced ferroptosis of IECs to improve RE.
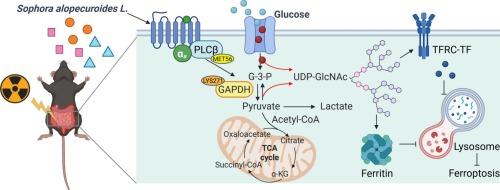
Aloperine通过驱动TAS2R138-PLCβ/GAPDH轴介导的糖代谢重编程来抑制肠上皮细胞的铁下垂,从而改善放射性肠炎
放射性肠炎(RE)是放射治疗的常见副作用,目前尚无专门的治疗药物。苦参(Sophora alopecuroides L., KDZ)在中国用于治疗胃肠道疾病已有多年历史,苦参碱、氧化苦参碱和苦参碱(ALO)是其主要生物碱成分。我们发现,ALO显著减弱了电离辐射(IR)对肠上皮细胞(IECs)活力的损害,优于苦参碱和氧化苦参碱。ir诱导的铁离子积累、脂质过氧化、谷胱甘肽过氧化物酶4和溶质载体家族7成员11水平下调恢复,并进一步表现为铁下垂,但未出现凋亡或坏死。非靶向代谢组学分析表明,ALO将葡萄糖通量从糖酵解转移到己糖胺生物合成途径,从而提高了UDP-GlcNAc水平。值得注意的是,ALO可以直接结合味觉2受体成员138 (TAS2R138)作为其潜在靶点之一,并促进下游信号分子磷脂酶Cβ (PLCβ)和α-gustducin的激活。此外,ALO增强了PLCβ和GAPDH在MET56和LYS271的关联,并通过激活TAS2R138抑制后者的乙酰化和活性。此外,在触发代谢重编程后,ALO促进O/ n糖基化,阻止铁蛋白重链1和转铁蛋白受体的溶酶体降解,调节铁的储存和运输,从而减轻铁凋亡。在体内,通过小鼠RE模型验证了ALO的上述作用及其机制。总的来说,ALO激活TAS2R138,促进PLCβ和GAPDH的相互作用,诱导代谢重编程,减轻ir诱导的IECs铁下沉,从而改善RE。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


