Transition Metal-Free γ-C(sp3)–H Functionalization of Enaminones for Methylene 1,2-Dihydropyridine Synthesis and Application in Fused Tricyclic Pyrido[1,2-a]indole Construction
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The annulation reaction of β-methyl enaminones with alkynones leading to the synthesis of methylene-functionalized 1,2-dihydropyridines (1,2-DHPs) has been realized. In the presence of only Cs2CO3, 1,2-DHPs have been synthesized with a broad scope and generally high efficiency via the novel transformation of the γ-C(sp3)–H bond in enaminones. In addition, the application of the method has been demonstrated by the one-step transformation of the 1,2-DHP products into fused tricyclic scaffolds featuring an indole–pyridine hybrid via dual C–H activation.

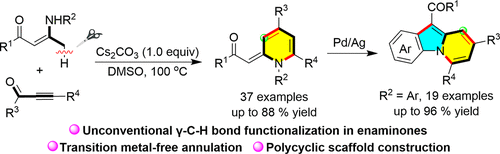
亚甲基1,2-二氢吡啶合成中无过渡金属γ-C(sp3) -H功能化胺酮及其在融合三环吡啶[1,2-a]吲哚结构中的应用
实现了β-甲基胺酮与炔酮的环化反应,合成了亚甲基功能化的1,2-二氢吡啶(1,2- dhps)。在仅存在Cs2CO3的情况下,通过γ-C(sp3) -H键的新型转化,合成了范围广、效率普遍较高的1,2- dhps。此外,通过双C-H活化将1,2- dhp产物一步转化为具有吲哚-吡啶杂化物的融合三环支架,证明了该方法的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


