Biochemical investigations into the incorporation of 8-oxo-2′-deoxyguanosine-5′-triphosphate with two A-family polymerases
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
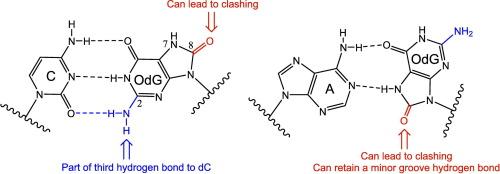
8-Oxo-2′-deoxyguanosine-5′-triphosphate (OdGTP) is a promutagenic oxidatively damaged nucleotide that can base pair to both 2′-deoxycytidine (dC) and 2′-deoxyadenosine (dA) and may play a role in antibiotic initiated bacterial cell death. We evaluated the insertion of OdGTP, dGTP, and eight related analogs opposite dC and dA with two A-family bacterial replicative polymerases, nuclease deficient Polymerase I from E. coli (KF-exo) and Geobacillus Stearothermophilus (BF). Results from these studies demonstrate that KF-exo is much less likely than BF to incorporate OdGTP opposite either dC or dA as compared to incorporation of dGTP opposite dC. Our work also highlights similarities and differences in the active sites that can occur between two polymerases within the same family. For example, the C2-amine of OdGTP appears to have little impact during incorporation opposite dA with either enzyme. However, when inserting opposite dC, the C2-amine can form a stabilizing hydrogen bond to the template dC, and increase activity, especially when large atoms are present at the C8-position. KF-exo is more sensitive than BF to large substituents at the C8-position during incorporation opposite dC, while BF, but not KF-exo, likely forms a minor groove hydrogen bond to the C8‑oxygen during incorporation opposite dA. Overall, these findings provide mechanistic insights into how oxidative stress can contribute to genomic instability through polymerase-mediated misincorporation events.
8-氧-2 ' -脱氧鸟苷-5 ' -三磷酸与两种a家族聚合酶结合的生化研究
8-氧-2 ' -脱氧鸟苷-5 ' -三磷酸(OdGTP)是一种促生性氧化损伤核苷酸,可与2 ' -脱氧胞苷(dC)和2 ' -脱氧腺苷(dA)碱基配对,可能在抗生素引发的细菌细胞死亡中起作用。我们评估了OdGTP、dGTP和8种与dC和dA相反的相关类似物与两种a家族细菌复制聚合酶的插入,即大肠杆菌(KF-exo)和嗜脂嗜热地杆菌(BF)的核酸酶缺陷聚合酶I。这些研究的结果表明,与dC对面的dGTP结合相比,KF-exo与dC对面的OdGTP结合的可能性要小得多。我们的工作还强调了同一家族中两种聚合酶活性位点的相似性和差异性。例如,OdGTP的c2 -胺在与相反的dA结合时似乎没有什么影响。然而,当插入相反的dC时,c2 -胺可以与模板dC形成稳定的氢键,并增加活性,特别是当c8位置存在大原子时。KF-exo对C8位置的大取代基比BF更敏感,而BF(而不是KF-exo)可能在与dA相反的方向结合时与C8-氧形成较小的凹槽氢键。总的来说,这些发现为氧化应激如何通过聚合酶介导的错误结合事件导致基因组不稳定提供了机制上的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


