Maternal exposure to advanced oxidation protein products induces neurodevelopmental abnormalities in the offspring of mice
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Preeclampsia (PE) not only causes multi-organ damage to the mother but also exerts long-term effects on the development of the offspring. Prenatal exposure to PE has been demonstrated to result in deleterious neurodevelopment and behavioral abnormalities in the offspring. Our prior research has evidenced that advanced oxidation protein products (AOPPs), both the biomarker and promoter of oxidative stress, contribute to the pathogenesis of preeclampsia by inducing trophoblast dysfunction and systemic inflammation. However, it remains unclear whether offspring mice exposed to placental oxidative stress and dysfunction induced by AOPPs will exhibit abnormal neurodevelopment and the underlying mechanisms involved. In this study, we found pregnant mice injected with AOPPs and AOPPs-induced sEVs exhibited PE-like symptoms with elevated blood pressure and reduced fetal weight. Furthermore, behavioral detection showed that male and female adult offspring mice exposed to AOPPs prenatally displayed impairments in learning and memory. Placental and hippocampal transcriptome sequencing revealed that inflammation and immune response may play a pivotal role in this process. Our study provides valuable insights into the pathogenesis of adverse neurodevelopment and cognitive dysfunction in offspring exposed to placental oxidative stress induced by AOPPs and the search for effective preventive and therapeutic measures.
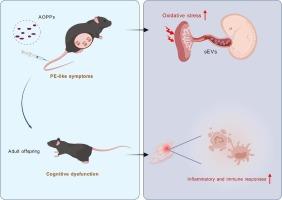
母体暴露于高级氧化蛋白产品诱导小鼠后代神经发育异常
子痫前期不仅对母体造成多器官损害,而且对子代发育有长期影响。产前暴露于PE已被证明会导致后代有害的神经发育和行为异常。我们之前的研究已经证明,高级氧化蛋白产物(AOPPs)作为氧化应激的生物标志物和启动子,通过诱导滋养细胞功能障碍和全身性炎症参与子痫前期的发病机制。然而,尚不清楚暴露于AOPPs诱导的胎盘氧化应激和功能障碍的后代小鼠是否会表现出异常的神经发育及其潜在机制。在本研究中,我们发现注射AOPPs和AOPPs诱导的sev的妊娠小鼠表现出pe样症状,血压升高,胎儿体重降低。此外,行为检测显示,暴露于AOPPs的雄性和雌性成年后代小鼠在产前表现出学习和记忆障碍。胎盘和海马转录组测序显示,炎症和免疫反应可能在这一过程中起关键作用。我们的研究提供了有价值的见解,揭示了暴露于AOPPs诱导的胎盘氧化应激的后代不良神经发育和认知功能障碍的发病机制,并寻求有效的预防和治疗措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


